Temperature coefficient of seawater pH as a function of temperature, pH, DIC and salinity
-
Abstract: pH is a measure of the hydrogen ion activity in a solution, which is a function of temperature. Under normal seawater conditions, it is well constrained. Nowadays, with an increasing interest in complex environments (e.g., sea ice), a better understanding of the temperature change on pH under extreme conditions is needed. The objective of this paper was to investigate the temperature coefficient of the seawater pH (∆pH/∆T) over a wide range of temperature, pH, dissolved inorganic carbon (DIC) and salinity by a method of continuous pH measurement with the temperature change, and to verify the application of CO2SYS for pH conversion under extreme conditions (on the National Bureau of Standards (NBS) scale and the total proton scale). Both experimental results and CO2SYS calculations showed that ∆pH/∆T was slightly affected by temperature over the range of 0°C to 40°C and by pH (at 25°C) from 7.8 to 8.5. However, when pH was out of this range, ∆pH/∆T varied greatly with pH value. According to the experimental results, changes in DIC from 1 mmol/kg to 5 mmol/kg and salinity from 20 to 105 had no significant effect on ∆pH/∆T. CO2SYS calculations showed a slight increase in ∆pH/∆T with DIC on both the NBS scale and the total proton scale; and underestimated ∆pH/∆T at high salinity (i.e., beyond the oceanographic range) on the NBS scale. Nevertheless, CO2SYS is still suitable for pH conversion even under extreme conditions by simply setting the input values of DIC and salinity in CO2SYS within the oceanographic range (e.g., DIC=2 mmol/kg and S=35).
-
Key words:
- temperature coefficient /
- pH measurement /
- CO2SYS /
- sea ice /
- ocean acidification
-
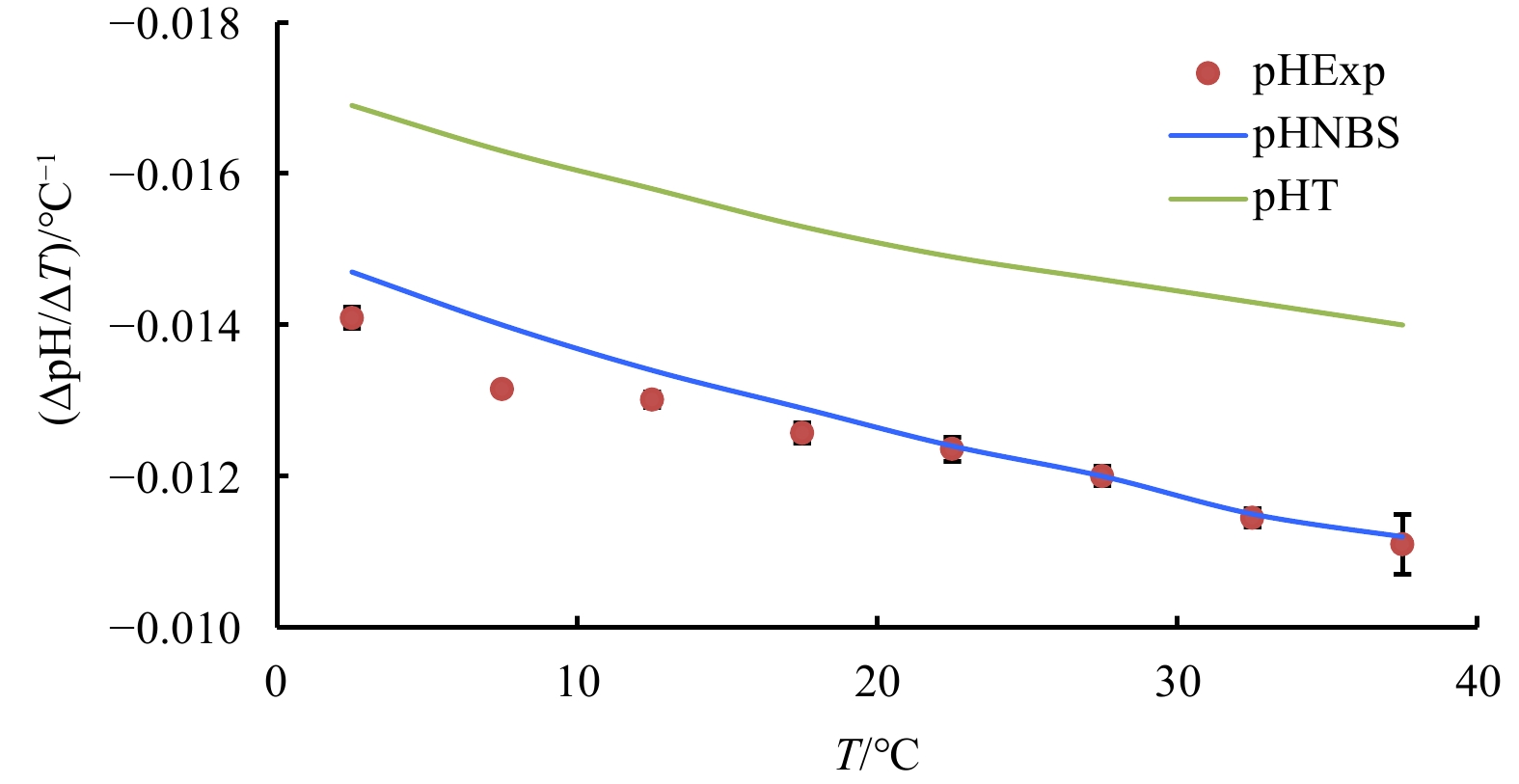
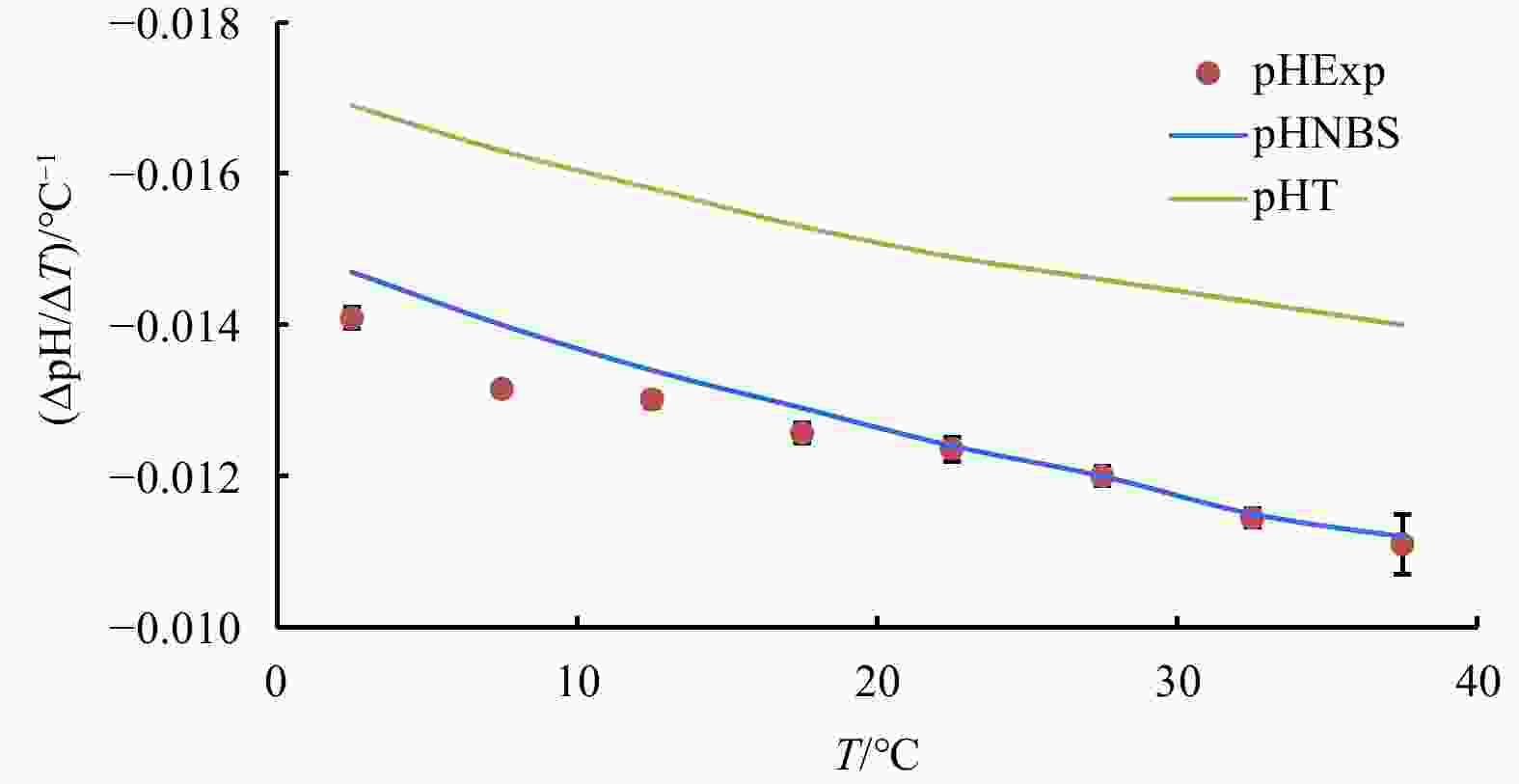
Figure 1. Temperature coefficient of seawater pH (∆pH/∆T) at every 5°C span from temperature 0°C to 40°C and at the experimental condition of salinity=35, DIC concentration=2 mmol/kg and pH=8.0 (25°C): experimental results are labelled by red dots; lines are derived from CO2SYS calculation based on the NBS scale (blue) and the total proton scale (green), respectively.
Figure 2. Temperature coefficient of seawater pH (∆pH/∆T) at different pH (25°C) from 7.5 to 10.0 and at the experimental condition of S=35, DIC concentration=2 mmol/kg: experimental results are labelled by red dots; lines are derived from CO2SYS calculation based on the NBS scale (blue) and the total proton scale (green), respectively. The uncertainty in ∆pH/∆T for each experimental data point is within ±0.000 2°C−1.
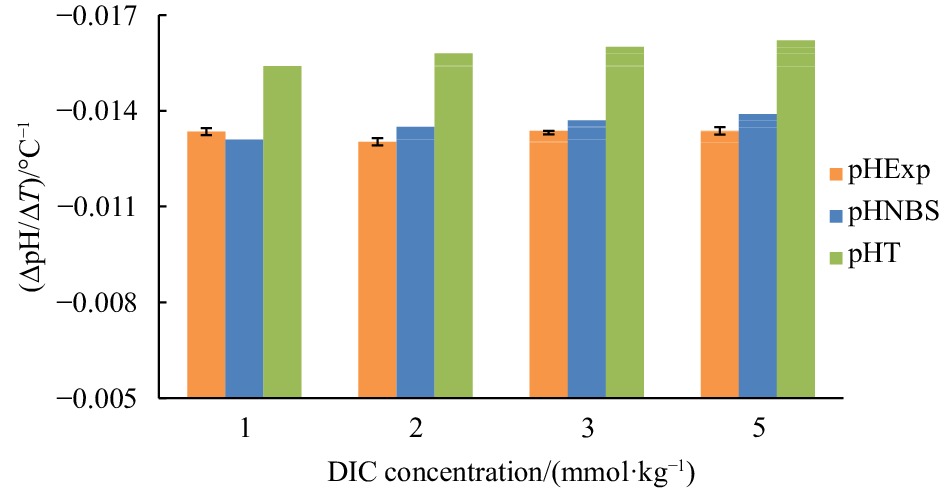
Figure 3. Temperature coefficient of seawater pH (∆pH/∆T) at different DIC concentrations from 1 mmol/kg to 5 mmol/kg and at the experimental condition of S=35, pH=8.0 (25oC): experimental results (orange), results derived from CO2SYS calculation based on the NBS scale (blue) and the total proton scale (green), respectively.
-
[1] Barron J J, Ashton C, Geary L. 2005. The effects of temperature on pH measurement. TSP-01, County Clare, Ireland: Technical Services Department, Reagecon Diagnostics Ltd, Shannon Free Zone, 1–7 [2] Ben-Yaakov S. 1970. A method for calculating the in situ pH of seawater. Limnology and Oceanography, 15(2): 326–328. doi: 10.4319/lo.1970.15.2.0326 [3] Dickson A G. 1990. Standard potential of the reaction: AgCl(s) + 1/2H2(g) = Ag(s) + HCl(aq), and the standard acidity constant of the ion ${\rm{HSO}}_4^− $ in synthetic sea water from 273.15 to 318.15 K. Journal of Chemical Thermodynamics, 22(2): 113–127. doi: 10.1016/0021-9614(90)90074-Z[4] Dickson A G, Millero F J. 1987. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Research Part A: Oceanographic Research Papers, 34(10): 1733–1743, [5] Dickson A G, Sabine C L, Christian J R E. 2007. Guide to best practices for ocean CO2 measurements. http://www.ioccp.org/index.php/documents/standards-and-methods/2-uncategorised/263-guide-to-best-practices-for-ocean-co2-measurements [2019-09-10] [6] Doney S C, Fabry V J, Feely R A, et al. 2009. Ocean acidification: the other CO2 problem. Annual Review of Marine Science, 1: 169–192. doi: 10.1146/annurev.marine.010908.163834 [7] Easley R A, Byrne R H. 2012. Spectrophotometric calibration of pH electrodes in seawater using purified m-cresol purple. Environmental Science & Technology, 46(9): 5018–5024. doi: 10.1021/es300491s [8] Fabry V J, McClintock J B, Mathis J T, et al. 2009. Ocean acidification at high latitudes: The bellwether. Oceanography, 22(4): 160–171. doi: 10.5670/oceanog.2009.105 [9] Feely R A, Doney S C, Cooley S R. 2009. Ocean acidification: Present conditions and future changes in a high-CO2 world. Oceanography, 22(4): 36–47. doi: 10.5670/oceanog.2009.95 [10] Gieskes J M. 1969. Effect of temperature on the pH of seawater. Limnology and Oceanography, 14(5): 679–685. doi: 10.4319/lo.1969.14.5.0679 [11] Gleitz M, Loeff M R V D, Thomas D N, et al. 1995. Comparison of summer and winter inorganic carbon, oxygen and nutrient concentrations in Antarctic sea ice brine. Marine Chemistry, 51(2): 81–91. doi: 10.1016/0304-4203(95)00053-T [12] Hare A A, Wang Feiyue, Barber D, et al. 2013. pH evolution in sea ice grown at an outdoor experimental facility. Marine Chemistry, 154: 46–54. doi: 10.1016/j.marchem.2013.04.007 [13] Hunter K A. 1998. The temperature dependence of pH in surface seawater. Deep-Sea Research Part I: Oceanographic Research Papers, 45(11): 1919–1930. doi: 10.1016/S0967-0637(98)00047-8 [14] IPCC. 2014. Climate Change 2013—The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 1535 [15] Kadis R, Leito I. 2010. Evaluation of the residual liquid junction potential contribution to the uncertainty in pH measurement: A case study on low ionic strength natural waters. Analytica Chimica Acta, 664(2): 129–135. doi: 10.1016/j.aca.2010.02.007 [16] Lui Hon-Kit, Chen Chen-Tung Arthur. 2017. Reconciliation of pH25 and pHinsitu acidification rates of the surface oceans: A simple conversion using only in situ temperature. Limnology and Oceanography: Methods, 15(3): 328–335. doi: 10.1002/lom3.10170 [17] Marion G M, Millero F J, Camães M F, et al. 2011. pH of seawater. Marine Chemistry, 126(1–4): 89–96. doi: 10.1016/j.marchem.2011.04.002 [18] Mehrbach C, Culberson C H, Hawley J E, et al. 1973. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnology and Oceanography, 18(6): 897–907. doi: 10.4319/lo.1973.18.6.0897 [19] Middelboe A L, Hansen P J. 2007. High pH in shallow-water macroalgal habitats. Marine Ecology Progress Series, 338: 107–117. doi: 10.3354/meps338107 [20] Millero F J. 1979. The thermodynamics of the carbonate system in seawater. Geochimica et Cosmochimica Acta, 43(10): 1651–1661. doi: 10.1016/0016–7037(79)90184–4 [21] Millero F J. 2006. Chemical Oceanography. 3rd ed. Boca Raton: CRC Press, 62 [22] Orr J C, Fabry V J, Aumont O, et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437(7059): 681–686. doi: 10.1038/nature04095 [23] Papadimitriou S, Loucaides S, Rérolle V, et al. 2016. The measurement of pH in saline and hypersaline media at sub-zero temperatures: Characterization of Tris buffers. Marine Chemistry, 184: 11–20. doi: 10.1016/j.marchem.2016.06.002 [24] Pierrot D E, Lewis E, Wallace D W R. 2006. MS Excel program developed for CO2 system calculations. Oak Ridge, Tennessee: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U. S. Department of Energy, [25] Steinacher M, Joos F, Frölicher T L, et al. 2009. Imminent ocean acidification in the Arctic projected with the NCAR global coupled carbon cycle-climate model. Biogeosciences, 6(4): 515–533. doi: 10.5194/bg-6-515-2009 [26] Uppström L R. 1974. The Boron/chlorinity ratio of deep-sea water from the Pacific Ocean. Deep-Sea Research and Oceanographic Abstracts, 21(2): 161–162. doi: 10.1016/0011-7471(74)90074-6 [27] Van Alstyne K L, Nelson T A, Ridgway R L. 2015. Environmental chemistry and chemical ecology of “green tide” seaweed blooms. Integrative and Comparative Biology, 55(3): 518–532. doi: 10.1093/icb/icv035 [28] Yamamoto-Kawai M, McLaughlin F A, Carmack E C, et al. 2009. Aragonite undersaturation in the Arctic Ocean: Effects of ocean acidification and sea ice melt. Science, 326(5956): 1098–1100. doi: 10.1126/science.1174190 -
 Hu Yubin Supplementary information.zip
Hu Yubin Supplementary information.zip

-



 下载:
下载: