-
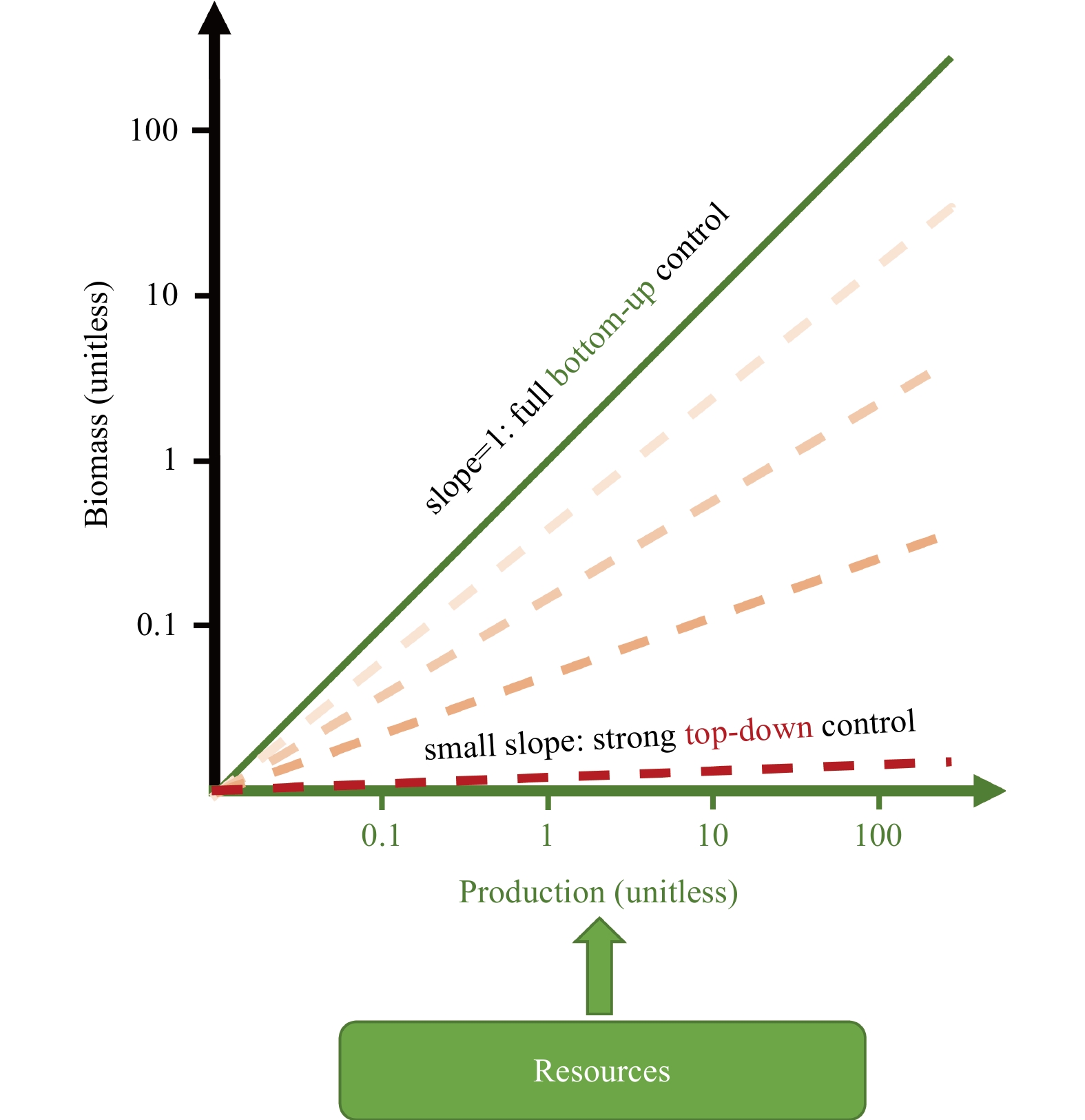
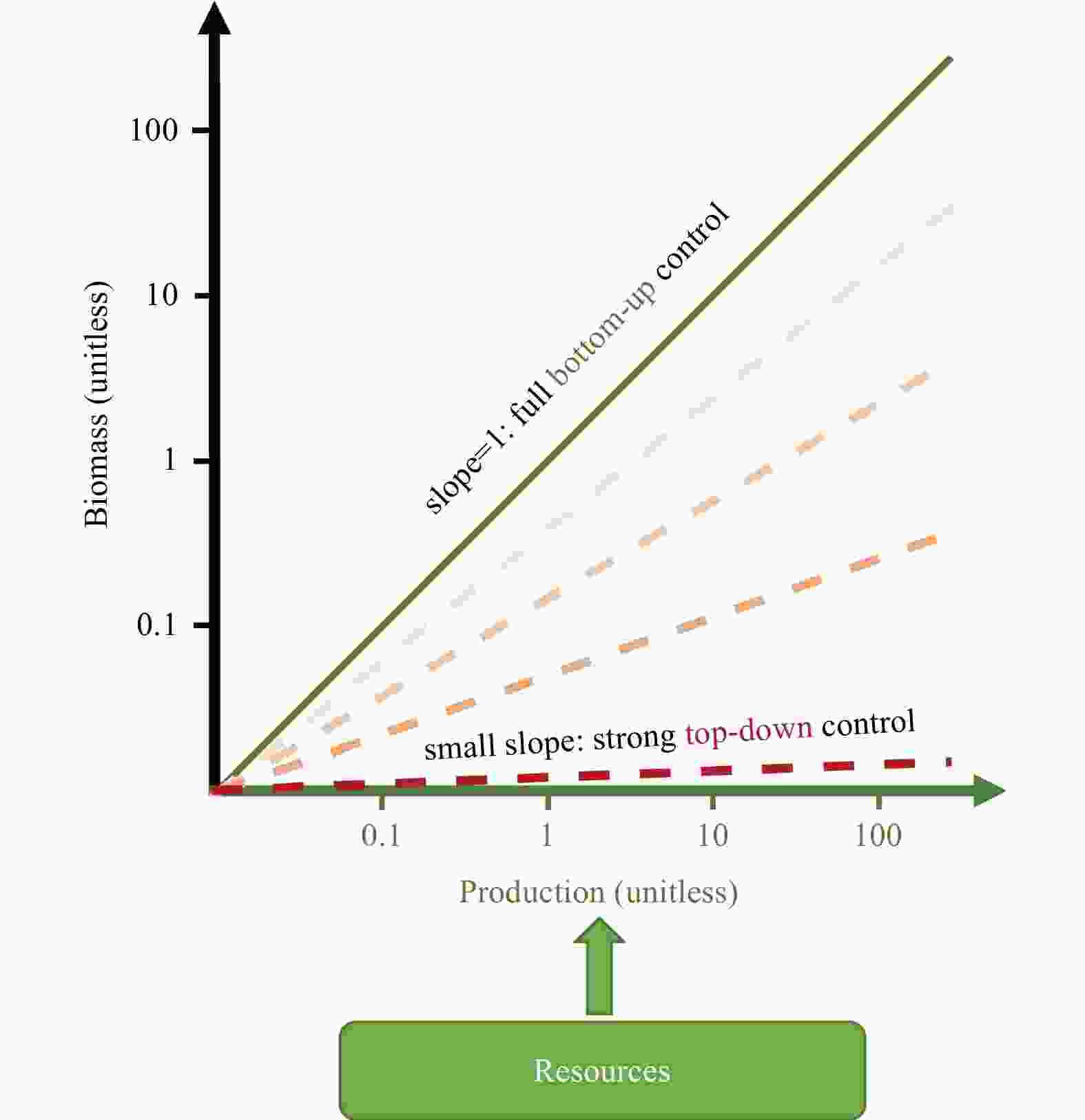
Abstract: Dinitrogen (N2) fixed by a group of prokaryotes (diazotrophs) is the dominant process adding bioavailable nitrogen into the ocean. Although it has been intensively studied how N2 fixation is controlled by resources (bottom-up factors), it is unclear whether the grazing (top-down control) effectively impacts growth and distribution of different diazotroph groups. In this study, we evaluate this question by conducting log-log regression of diazotroph biomass onto corresponding N2 fixation rates in the global ocean. The slope of the regression for Trichodesmium is ~0.8, indicating that a small portion of the increase in N2 fixation does not accumulate as its biomass. This leads to a conclusion that Trichodesmium is under a substantial top-down control, although bottom-up control still dominates. We also analyze the residuals of the regression in the North Atlantic, concluding that free trichomes of Trichodesmium are subject to stronger top-down control than its colonies. The weak correlation between the biomass and N2 fixation of unicellular cyanobacterial diazotrophs indicates that the degree of top-down control on this type of diazotrophs varies greatly. The analyses obtain unrealistic results for diatom-diazotroph assemblages due to complicated nitrogen sources of these symbioses. Our study reveals the variability of top-down control among different diazotroph groups across time and space, suggesting its importance in improving our understandings of ecology of diazotrophs and predictions of N2 fixation in biogeochemical models. Measurements of size-specific N2 fixation rates and growth rates of different diazotroph groups can be useful to more reliably analyze the top-down control on these key organisms in the global ocean.
-
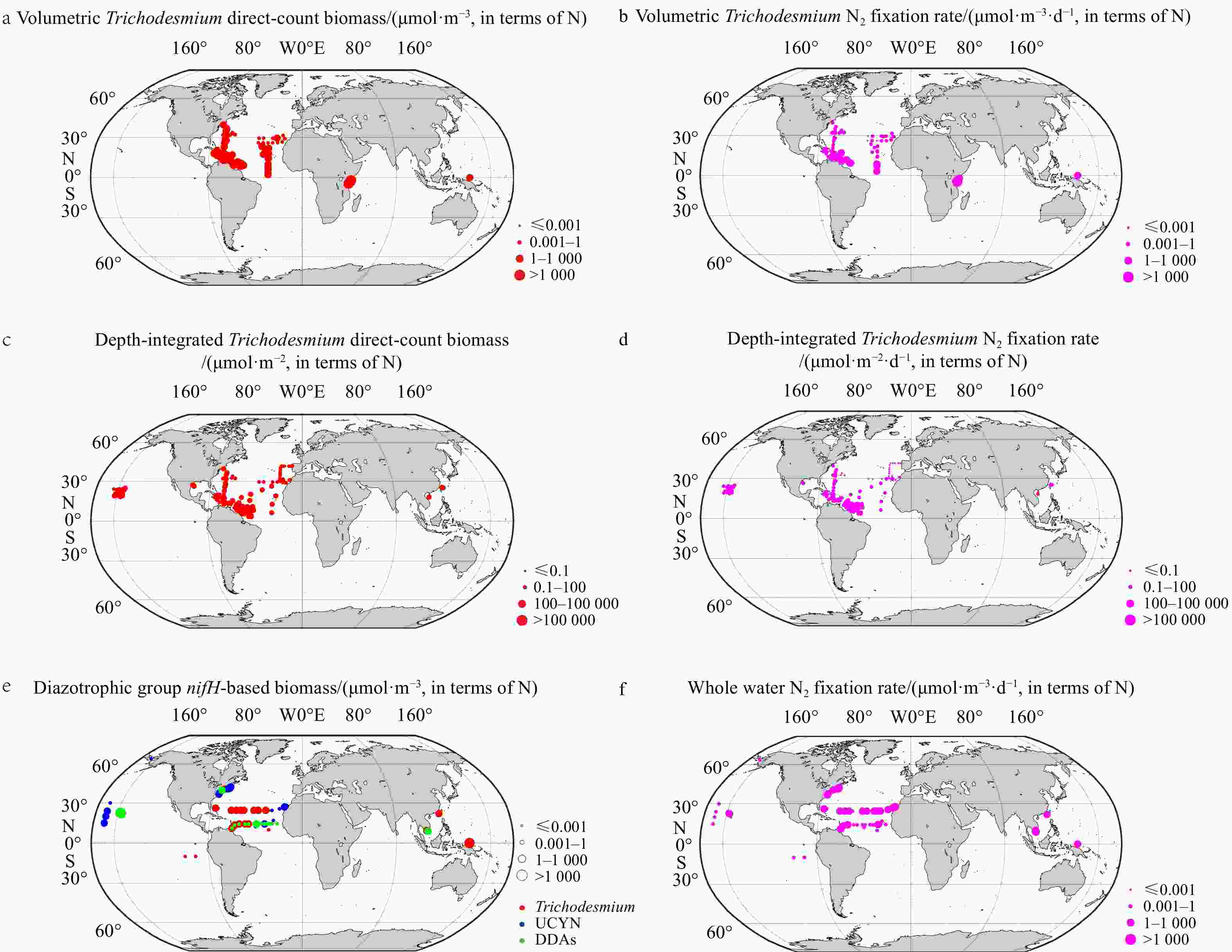
Figure 2. Spatial distributions of collected diazotrophic data. Volumetric (a) and depth-integrated (c) Trichodesmium biomass using directly counted abundance data. Diazotroph biomass derived from nifH-based abundance, with the color representing the dominant group (note that some data points overlap spatially) (e). In b, d and f, N2 fixation rates paired to diazotrophic biomass in a, c and e, respectively.
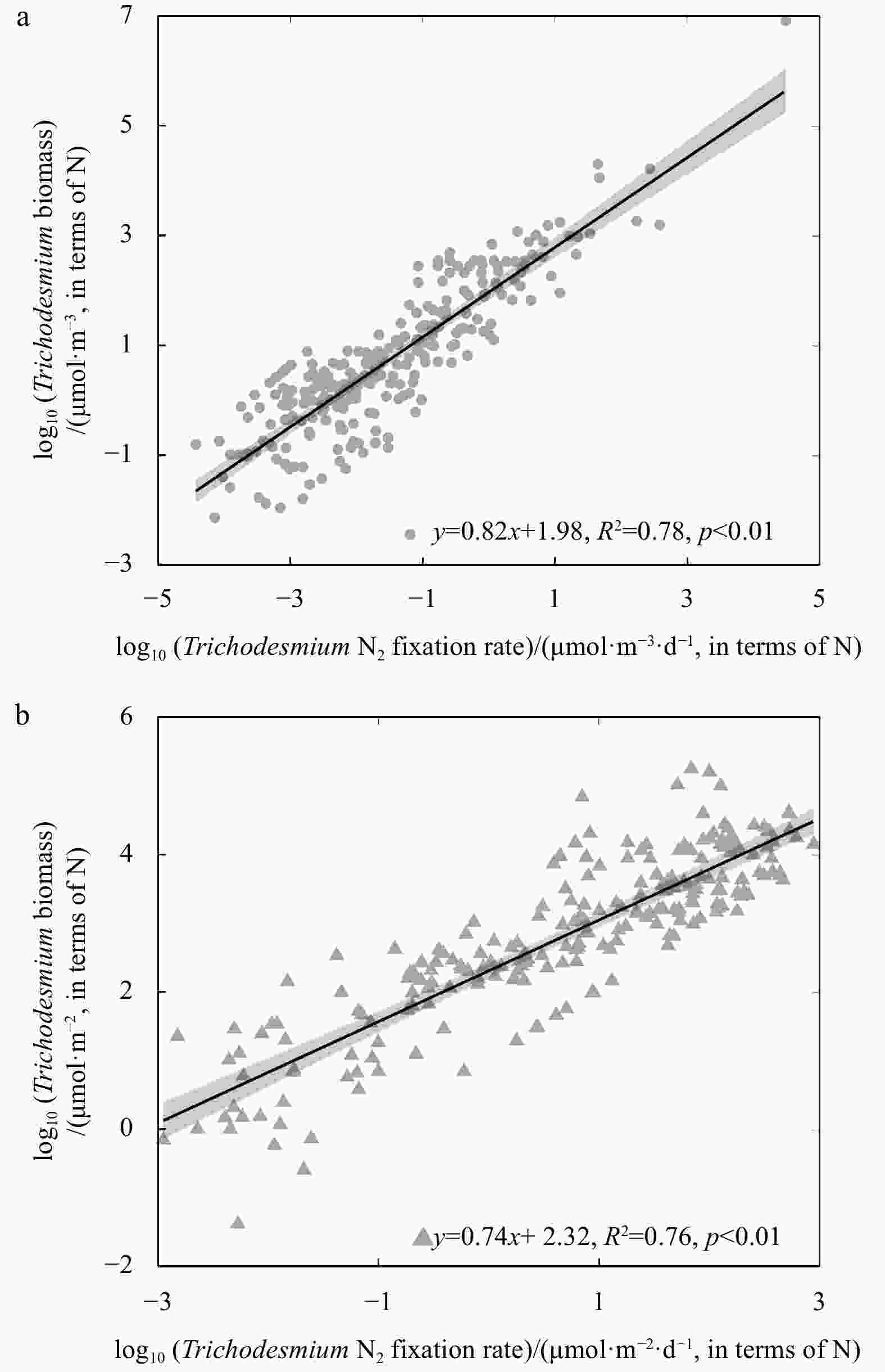
Figure 5. The log-log regression of nifH-based diazotroph biomass to N2 fixation rate. The data are separated into three categories according to dominant diazotroph group including Trichodesmium (red) (n=54), unicellular N2-fixing cyanobacteria (UCYN, blue) (n=30) and diatom-diazotroph assemblages (DDA, green) (n=32).
Table 1. Carbon biomass conversion factors from abundance
Type Diazotrophic group Conversion
factorUnit Direct
countTrichodesmium 300 pg/cell, in terms of C nifH Trichodesmium 30 pg C per nifH gene copy UCYN-A 2 pg C per nifH gene copy UCYN-B 20 pg C per nifH gene copy UCYN-C 10 pg C per nifH gene copy Richelia-Hemiaulus 43.5 pg C per nifH gene copy Richelia-Rhizosolenia 50.1 pg C per nifH gene copy -
[1] Agawin N S R, Benavides M, Busquets A, et al. 2014. Dominance of unicellular cyanobacteria in the diazotrophic community in the Atlantic Ocean. Limnology and Oceanography, 59(2): 623–637. doi: 10.4319/lo.2014.59.2.0623 [2] Barton A D, Finkel Z V, Ward B A, et al. 2013. On the roles of cell size and trophic strategy in North Atlantic diatom and dinoflagellate communities. Limnology and Oceanography, 58(1): 254–266. doi: 10.4319/lo.2013.58.1.0254 [3] Benavides M, Agawin N S R, Arístegui J, et al. 2011. Nitrogen fixation by Trichodesmium and small diazotrophs in the subtropical northeast Atlantic. Aquatic Microbial Ecology, 65(1): 43–53. doi: 10.3354/ame01534 [4] Benavides M, Moisander P H, Daley M C, et al. 2016. Longitudinal variability of diazotroph abundances in the subtropical North Atlantic Ocean. Journal of Plankton Research, 38(3): 662–672. doi: 10.1093/plankt/fbv121 [5] Billen G, Servais P, Becquevort S. 1990. Dynamics of bacterioplankton in oligotrophic and eutrophic aquatic environments: bottom-up or top-down control? Hydrobiologia, 207(1): 37–42,doi: 10.1007/BF00041438 [6] Bombar D, Paerl R W, Riemann L. 2016. Marine non-cyanobacterial diazotrophs: moving beyond molecular detection. Trends in Microbiology, 24(11): 916–927. doi: 10.1016/j.tim.2016.07.002 [7] Boyer T P, Garcia H E, Locarnini R A, et al. 2018. World Ocean Atlas 2018. NOAA National Centers for Environmental Information. https://accession.nodc.noaa.gov/NCEI-WOA18[2021-08-30] [8] Cabello A M, Cornejo-Castillo F M, Raho N, et al. 2016. Global distribution and vertical patterns of a prymnesiophyte-cyanobacteria obligate symbiosis. The ISME Journal, 10(3): 693–706. doi: 10.1038/ismej.2015.147 [9] Cáceres C, Taboada F G, Höfer J, et al. 2013. Phytoplankton growth and microzooplankton grazing in the subtropical northeast Atlantic. PLoS One, 8(7): e69159. doi: 10.1371/journal.pone.0069159 [10] Capone D G, Subramaniam A, Montoya J P, et al. 1998. An extensive bloom of the N2-fixing cyanobacterium Trichodesmium erythraeum in the central Arabian Sea. Marine Ecology Progress Series, 172: 281–292. doi: 10.3354/meps172281 [11] Capone D G, Zehr J P, Paerl H W, et al. 1997. Trichodesmium, a globally significant marine cyanobacterium. Science, 276(5316): 1221–1229. doi: 10.1126/science.276.5316.1221 [12] Carpenter E J, Capone D G, Rueter J R. 1992. Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs. Dordrecht: Springer [13] Conroy B J, Steinberg D K, Song B, et al. 2017. Mesozooplankton graze on cyanobacteria in the amazon river plume and western tropical North Atlantic. Frontiers in Microbiology, 8: 1436. doi: 10.3389/fmicb.2017.01436 [14] Cornejo-Castillo F M, Cabello A M, Salazar G, et al. 2016. Cyanobacterial symbionts diverged in the late Cretaceous towards lineage-specific nitrogen fixation factories in single-celled phytoplankton. Nature Communications, 7: 11071. doi: 10.1038/ncomms11071 [15] Cornejo-Castillo F M, del Carmen Muñoz-Marín M, Turk-Kubo K A, et al. 2019. UCYN-A3, a newly characterized open ocean sublineage of the symbiotic N2-fixing cyanobacterium Candidatus Atelocyanobacterium thalassa. Environmental Microbiology, 21(1): 111–124. doi: 10.1111/1462-2920.14429 [16] Dekaezemacker J, Bonnet S. 2011. Sensitivity of N2 fixation to combined nitrogen forms ( $\text{NO}_3^− $ and$\text{NH}_4^+ $ ) in two strains of the marine diazotroph Crocosphaera watsonii (Cyanobacteria). Marine Ecology Progress Series, 438: 33–46. doi: 10.3354/meps09297[17] Detoni A M S, Costa L D F, Pacheco L A, et al. 2016. Toxic Trichodesmium bloom occurrence in the southwestern South Atlantic Ocean. Toxicon, 110: 51–55. doi: 10.1016/j.toxicon.2015.12.003 [18] Dufour P H, Torréton J P. 1996. Bottom-up and top-down control of bacterioplankton from eutrophic to oligotrophic sites in the tropical northeastern Atlantic Ocean. Deep-Sea Research Part I: Oceanographic Research Papers, 43(8): 1305–1320. doi: 10.1016/0967-0637(96)00060-X [19] Dugenne M, Henderikx Freitas F, Wilson S T, et al. 2020. Life and death of Crocosphaera sp. in the Pacific Ocean: Fine scale predator–prey dynamics. Limnology and Oceanography, 65(11): 2603–2617. doi: 10.1002/lno.11473 [20] Falkowski P G, Koblfzek M, Gorbunov M, et al. 2004. Development and application of variable chlorophyll fluorescence techniques in marine ecosystems. In: Papageorgiou G C, Govindjee, eds. Chlorophyll a Fluorescence: A Signature of Photosynthesis. Dordrecht: Springer, 757–778 [21] Fonseca-Batista D, Li X F, Riou V, et al. 2019. Evidence of high N2 fixation rates in the temperate northeast Atlantic. Biogeosciences, 16(5): 999–1017. doi: 10.5194/bg-16-999-2019 [22] Foster R A, Kuypers M M M, Vagner T, et al. 2011. Nitrogen fixation and transfer in open ocean diatom-cyanobacterial symbioses. The ISME Journal, 5(9): 1484–1493. doi: 10.1038/ismej.2011.26 [23] García-Gómez C, Mata M T, Van Breusegem F, et al. 2016. Low-steady-state metabolism induced by elevated CO2 increases resilience to UV radiation in the unicellular green-algae Dunaliella tertiolecta. Environmental and Experimental Botany, 132: 163–174. doi: 10.1016/j.envexpbot.2016.09.001 [24] Gruber N. 2008. The marine nitrogen cycle: overview and challenges. In: Capone D G, Bronk D A, Mulholland M R, et al., eds. Nitrogen in the Marine Environment. 2nd ed. San Diego: Academic Press, 1–50 [25] Guo C Z, Tester P A. 1994. Toxic effect of the bloom-forming Trichodesmium sp. (cyanophyta) to the copepod Acartia tonsa. Natural Toxins, 2(4): 222–227. doi: 10.1002/nt.2620020411 [26] Hawser S P, O′Neil J M, Roman M R, et al. 1992. Toxicity of blooms of the cyanobacterium Trichodesmium to zooplankton. Journal of Applied Phycology, 4(1): 79–86. doi: 10.1007/BF00003963 [27] Holl C M, Montoya J P. 2005. Interactions between nitrate uptake and nitrogen fixation in continuous cultures of the marine diazotroph Trichodesmium (cyanobacteria). Journal of Phycology, 41(6): 1178–1183. doi: 10.1111/j.1529-8817.2005.00146.x [28] Holl C M, Villareal T A, Payne C D, et al. 2007. Trichodesmium in the western Gulf of Mexico: 15N2-fixation and natural abundance stable isotopic evidence. Limnology and Oceanography, 52(5): 2249–2259. doi: 10.4319/lo.2007.52.5.2249 [29] Hunt B P V, Bonnet S, Berthelot H, et al. 2016. Contribution and pathways of diazotroph-derived nitrogen to zooplankton during the VAHINE mesocosm experiment in the oligotrophic New Caledonia Lagoon. Biogeosciences, 13(10): 3131–3145. doi: 10.5194/bg-13-3131-2016 [30] Karl D M, Church M J, Dore J E, et al. 2012. Predictable and efficient carbon sequestration in the North Pacific Ocean supported by symbiotic nitrogen fixation. Proceedings of the National Academy of Sciences of the United States of America, 109(6): 1842–1849. doi: 10.1073/pnas.1120312109 [31] Karl D, Michaels A, Bergman B, et al. 2002. Dinitrogen fixation in the world′s oceans. Biogeochemistry, 57–58: 47–98, [32] Keller D P, Oschlies A, Eby M. 2012. A new marine ecosystem model for the University of Victoria Earth System Climate Model. Geoscientific Model Development, 5(5): 1195–1220. doi: 10.5194/gmd-5-1195-2012 [33] LaRoche J, Breitbarth E. 2005. Importance of the diazotrophs as a source of new nitrogen in the ocean. Journal of Sea Research, 53(1–2): 67–91. doi: 10.1016/j.seares.2004.05.005 [34] Liu Hongbin, Buskey E J. 2000. The exopolymer secretions (EPS) layer surrounding Aureoumbra lagunensis cells affects growth, grazing, and behavior of protozoa. Limnology and Oceanography, 45(5): 1187–1191. doi: 10.4319/lo.2000.45.5.1187 [35] Lugomela C, Lyimo T J, Bryceson I, et al. 2002. Trichodesmium in coastal waters of Tanzania: diversity, seasonality, nitrogen and carbon fixation. Hydrobiologia, 477(1–3): 1–13. doi: 10.1023/A:1021017125376 [36] Luo Ya-Wei, Doney S C, Anderson L A, et al. 2012. Database of diazotrophs in global ocean: abundance, biomass and nitrogen fixation rates. Earth System Science Data, 4(1): 47–73. doi: 10.5194/essd-4-47-2012 [37] Luo Ya-Wei, Lima I D, Karl D M, et al. 2014. Data-based assessment of environmental controls on global marine nitrogen fixation. Biogeosciences, 11(3): 691–708. doi: 10.5194/bg-11-691-2014 [38] Moisander P H, Beinart R A, Hewson I, et al. 2010. Unicellular cyanobacterial distributions broaden the oceanic N2 fixation domain. Science, 327(5972): 1512–1514. doi: 10.1126/science.1185468 [39] Montoya J P, Carpenter E J, Capone D G. 2002. Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnology and Oceanography, 47(6): 1617–1628. doi: 10.4319/lo.2002.47.6.1617 [40] Mulholland M R. 2007. The fate of nitrogen fixed by diazotrophs in the ocean. Biogeosciences, 4(1): 37–51. doi: 10.5194/bg-4-37-2007 [41] Neuer S, Cianca A, Helmke P, et al. 2007. Biogeochemistry and hydrography in the eastern subtropical North Atlantic gyre. Results from the European time-series station ESTOC. Progress in Oceanography, 72(1): 1–29. doi: 10.1016/j.pocean.2006.08.001 [42] O′Neil J M, Metzler P M, Glibert P M. 1996. Ingestion of 15N2-labelled Trichodesmium spp. and ammonium regeneration by the harpacticoid copepod Macrosetella gracilis. Marine Biology, 125(1): 89–96. doi: 10.1007/BF00350763 [43] Pace M L, Cole J J. 1994. Comparative and experimental approaches to top-down and bottom-up regulation of bacteria. Microbial Ecology, 28(2): 181–193. doi: 10.1007/BF00166807 [44] Paulsen H, Ilyina T, Six K D, et al. 2017. Incorporating a prognostic representation of marine nitrogen fixers into the global ocean biogeochemical model HAMOCC. Journal of Advances in Modeling Earth Systems, 9(1): 438–464. doi: 10.1002/2016ms000737 [45] Raveh O, David N, Rilov G, et al. 2015. The temporal dynamics of coastal phytoplankton and bacterioplankton in the eastern Mediterranean Sea. PLoS ONE, 10(10): e0140690. doi: 10.1371/journal.pone.0140690 [46] Scavotto R E, Dziallas C, Bentzon-Tilia M, et al. 2015. Nitrogen-fixing bacteria associated with copepods in coastal waters of the North Atlantic Ocean. Environmental Microbiology, 17(10): 3754–3765. doi: 10.1111/1462-2920.12777 [47] Sheridan C C, Steinberg D K, Kling G W. 2002. The microbial and metazoan community associated with colonies of Trichodesmium spp. : a quantitative survey. Journal of Plankton Research, 24(9): 913–922. doi: 10.1093/plankt/24.9.913 [48] Siegel D A, Buesseler K O, Behrenfeld M J, et al. 2016. Prediction of the export and fate of global ocean net primary production: The EXPORTS science plan. Frontiers in Marine Science, 3: 22. doi: 10.3389/fmars.2016.00022 [49] Siegel D A, Buesseler K O, Doney S C, et al. 2014. Global assessment of ocean carbon export by combining satellite observations and food-web models. Global Biogeochemical Cycles, 28(3): 181–196. doi: 10.1002/2013gb004743 [50] Singh A, Gandhi N, Ramesh R. 2019. Surplus supply of bioavailable nitrogen through N2 fixation to primary producers in the eastern Arabian Sea during autumn. Continental Shelf Research, 181: 103–110. doi: 10.1016/j.csr.2019.05.012 [51] Sohm J A, Edwards B R, Wilson B G, et al. 2011. Constitutive extracellular polysaccharide (EPS) production by specific isolates of Crocosphaera watsonii. Frontiers in Microbiology, 2: 229. doi: 10.3389/fmicb.2011.00229 [52] Stukel M R, Coles V J, Brooks M T, et al. 2014. Top-down, bottom-up and physical controls on diatom-diazotroph assemblage growth in the Amazon River plume. Biogeosciences, 11(12): 3259–3278. doi: 10.5194/bg-11-3259-2014 [53] Tang Weiyi, Cassar N. 2019. Data-driven modeling of the distribution of diazotrophs in the global ocean. Geophysical Research Letters, 46(21): 12258–12269. doi: 10.1029/2019gl084376 [54] Tang Weiyi, Li Zuchuan, Cassar N. 2019. Machine learning estimates of global marine nitrogen fixation. Journal of Geophysical Research, 124(3): 717–730. doi: 10.1029/2018JG004828 [55] Thompson A, Carter B J, Turk-Kubo K, et al. 2014. Genetic diversity of the unicellular nitrogen-fixing cyanobacteria UCYN-A and its prymnesiophyte host. Environmental Microbiology, 16(10): 3238–3249. doi: 10.1111/1462-2920.12490 [56] Thompson A W, Foster R A, Krupke A, et al. 2012. Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science, 337(6101): 1546–1550. doi: 10.1126/science.1222700 [57] Tyrrell T. 1999. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature, 400(6744): 525–531. doi: 10.1038/22941 [58] Verity P G, Robertson C Y, Tronzo C R, et al. 1992. Relationships between cell volume and the carbon and nitrogen content of marine photosynthetic nanoplankton. Limnology and Oceanography, 37(7): 1434–1446. doi: 10.4319/lo.1992.37.7.1434 [59] Villareal T A. 1992. Marine nitrogen-fixing diatom-cyanobacteria symbioses. In: Carpenter E J, Capone D G, Rueter J G, eds. Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs. Dordrecht: Springer, 163–175 [60] Wang Weilei, Moore J K, Martiny A C, et al. 2019. Convergent estimates of marine nitrogen fixation. Nature, 566(7743): 205–211. doi: 10.1038/s41586-019-0911-2 [61] Webb E A, Ehrenreich I M, Brown S L, et al. 2009. Phenotypic and genotypic characterization of multiple strains of the diazotrophic cyanobacterium, Crocosphaera watsonii, isolated from the open ocean. Environmental Microbiology, 11(2): 338–348. doi: 10.1111/j.1462-2920.2008.01771.x [62] White A E, Watkins-Brandt K S, Church M J. 2018. Temporal variability of Trichodesmium spp. and diatom-diazotroph assemblages in the North Pacific subtropical gyre. Frontiers in Marine Science, 5: 27. doi: 10.3389/fmars.2018.00027 [63] Wilson S T, Aylward F O, Ribalet F, et al. 2017. Coordinated regulation of growth, activity and transcription in natural populations of the unicellular nitrogen-fixing cyanobacterium Crocosphaera. Nature Microbiology, 2(9): 17118. doi: 10.1038/nmicrobiol.2017.118 [64] Yeung L Y, Berelson W M, Young E D, et al. 2012. Impact of diatom-diazotroph associations on carbon export in the Amazon River plume. Geophysical Research Letters, 39(18): L18609. doi: 10.1029/2012GL053356 [65] Zehr J P. 2011. Nitrogen fixation by marine cyanobacteria. Trends in Microbiology, 19(4): 162–173. doi: 10.1016/j.tim.2010.12.004 [66] Zehr J P, Capone D G. 2020. Changing perspectives in marine nitrogen fixation. Science, 368(6492): eaay9514. doi: 10.1126/science.aay9514 [67] Zehr J P, Kudela R M. 2010. Nitrogen cycle of the open ocean: from genes to ecosystems. Annual Review of Marine Science, 3: 197–225. doi: 10.1146/annurev-marine-120709-142819 -



 下载:
下载: