Spatial and diel variations of the prokaryotic community in the Phaeocystis globosa blooms area of Beibu Gulf, China
-
Abstract: While prokaryotes play key roles in nutrient cycling and energy flow during Phaeocystis globosa blooms, the information on the spatial and diel temporal distribution of the prokaryotic community during Phaeocystis blooms remains scarce. In January 2019, we used high-throughput sequencing of the 16S rRNA gene to explore the spatial and diel variations of particle-attached (PA) and free-living (FL) prokaryotic communities during the blooming phase of P. globosa in Beibu Gulf, Guangxi, China. The results suggested a significant spatial variation pattern in the horizontal distribution of prokaryotic communities, while there was no significant difference in the vertical direction. Both spatial distance and environmental variables shaped the horizontal distribution of the prokaryotic community structure, while environmental variables, particularly the abundance of P. globosa colony and Chl a, showed more significant influence and were closely related to the structure and variation of the prokaryotic community. Strong vertical mixing of the water column disrupted the vertical structure heterogeneity of the prokaryotic community in winter. There were significant differences in the diel samples of PA prokaryotic communities, but not in the FL prokaryotic communities. Nitrate, ammonium and the abundance of P. globosa colony were the key environmental variables impacting the diel variations of prokaryotic communities over the sampling period. The present study provided valuable information to depict the spatial-temporal variations of the microbial community and its association with environmental parameters during P. globosa bloom in the tropical gulf.
-
Key words:
- algal bloom /
- 16S rRNA genes /
- prokaryotic /
- community diversity /
- spatial patterns /
- diel distribution
-
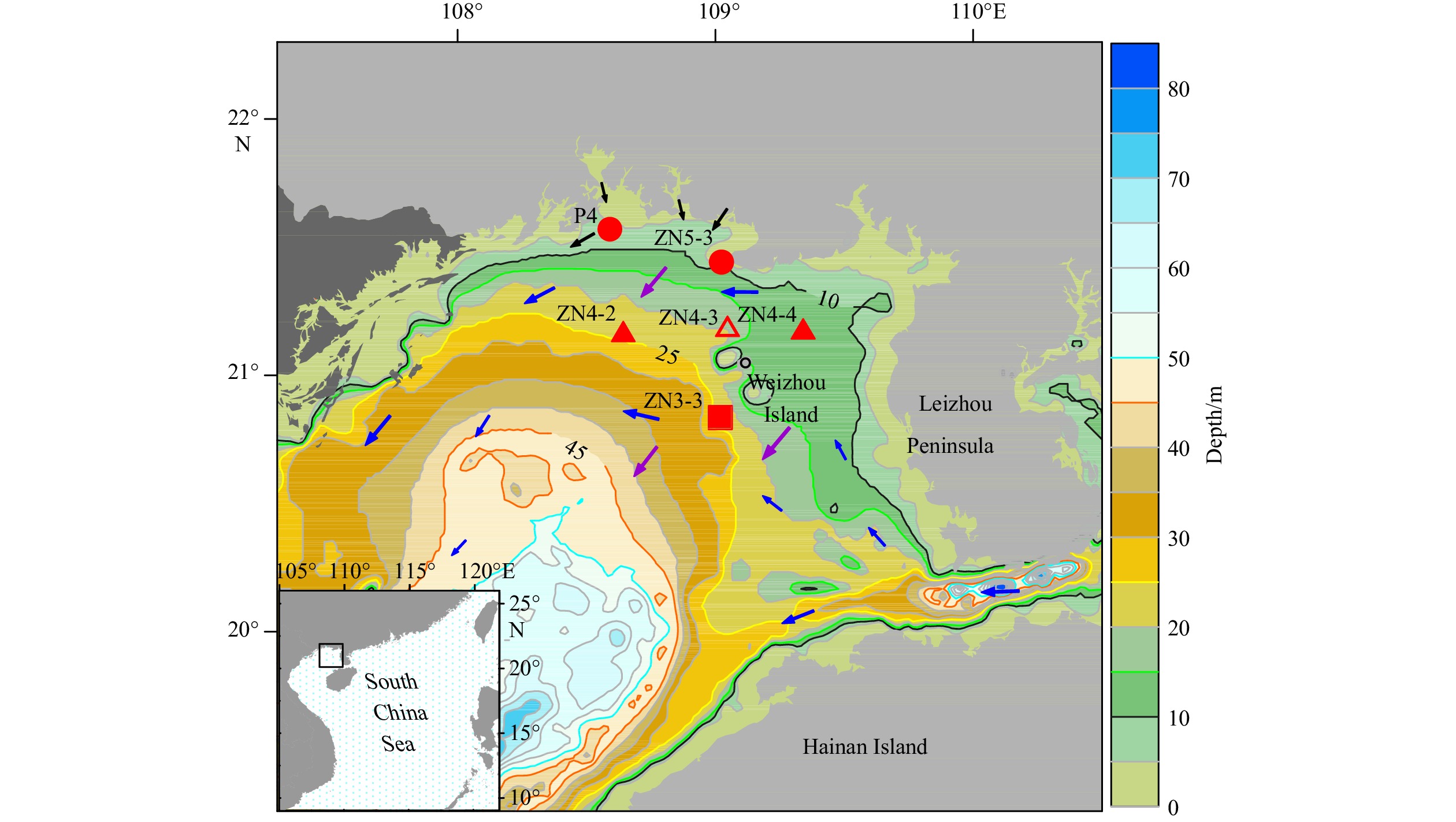
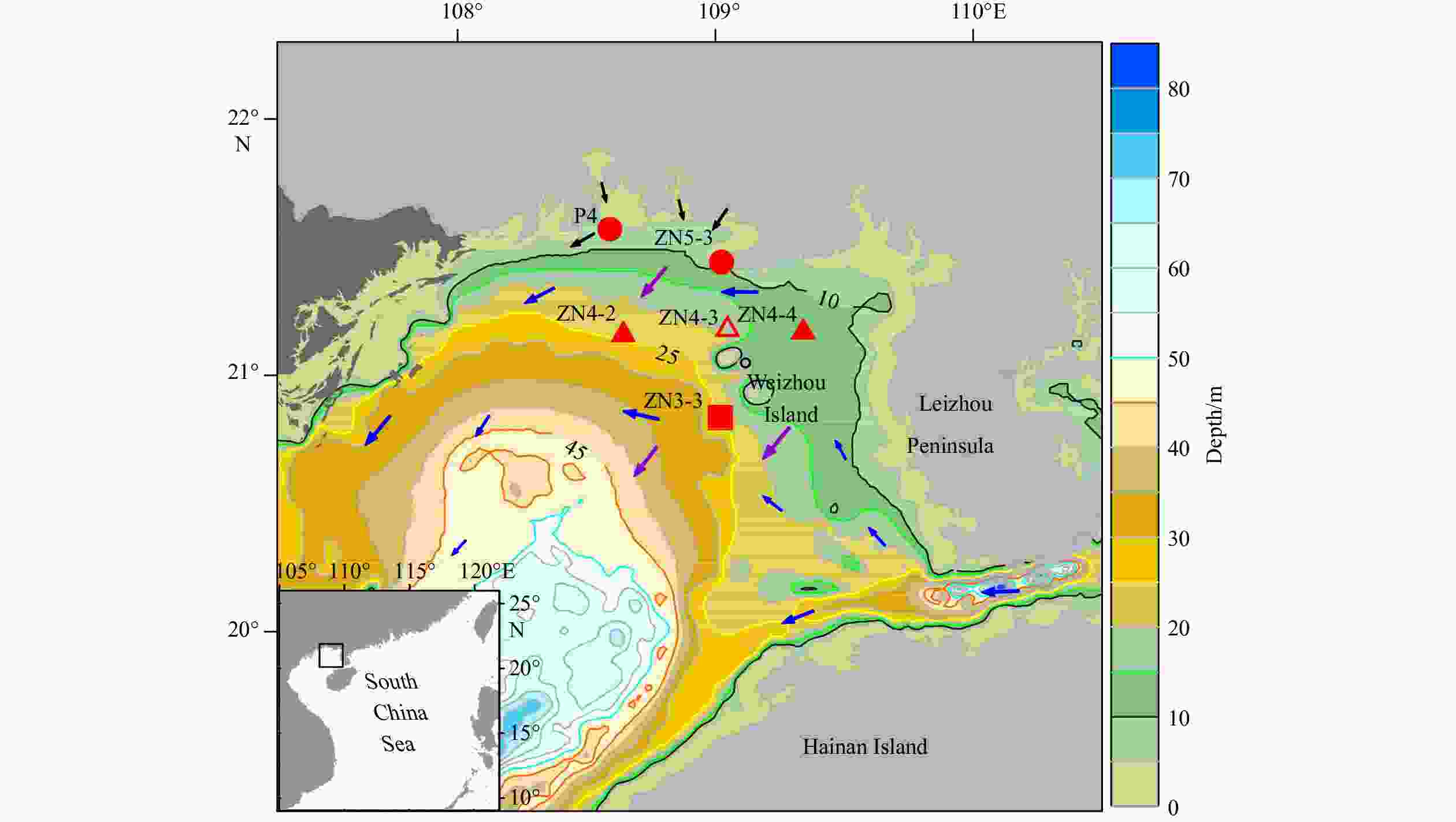
Figure 1. Location of sampling stations in the northern Beibu Gulf, China. The circles represent coastal stations, the square represents the deeper water station, and the triangles represent stations of the ZN4-X section, among which the empty triangle indicates the diel sampling station. Blue arrows indicate the general pattern of cyclonic circulation, purple arrows show the northeastern monsoon in winter, and black arrows point out riverine inputs in the sampling area (Bauer et al., 2013; Chen, 2013).
Figure 2. The heatmap of prokaryotic community composition in spatial (a) and diel (b) samples at the family level. The information of samples and species annotation were demonstrated along X-axis and Y-axis, respectively. The clustering tree was generated based on the family with the relative abundance >1.0% in at least one sample. The relative values in the heatmap depicted by colors after normalization indicated the aggregation degree or content of prokaryotic species among samples at the family level. Prefix: J, January. Suffix: BA, bottom particle-attached prokaryotes; SA, surface particle-attached prokaryotes; BF, bottom free-living prokaryotes; SF, surface free-living prokaryotes; SNA, surface night particle-attached prokaryotes; SDA, surface day particle-attached prokaryotes; SNF, surface night free-living prokaryotes; SDF, surface day free-living prokaryotes.
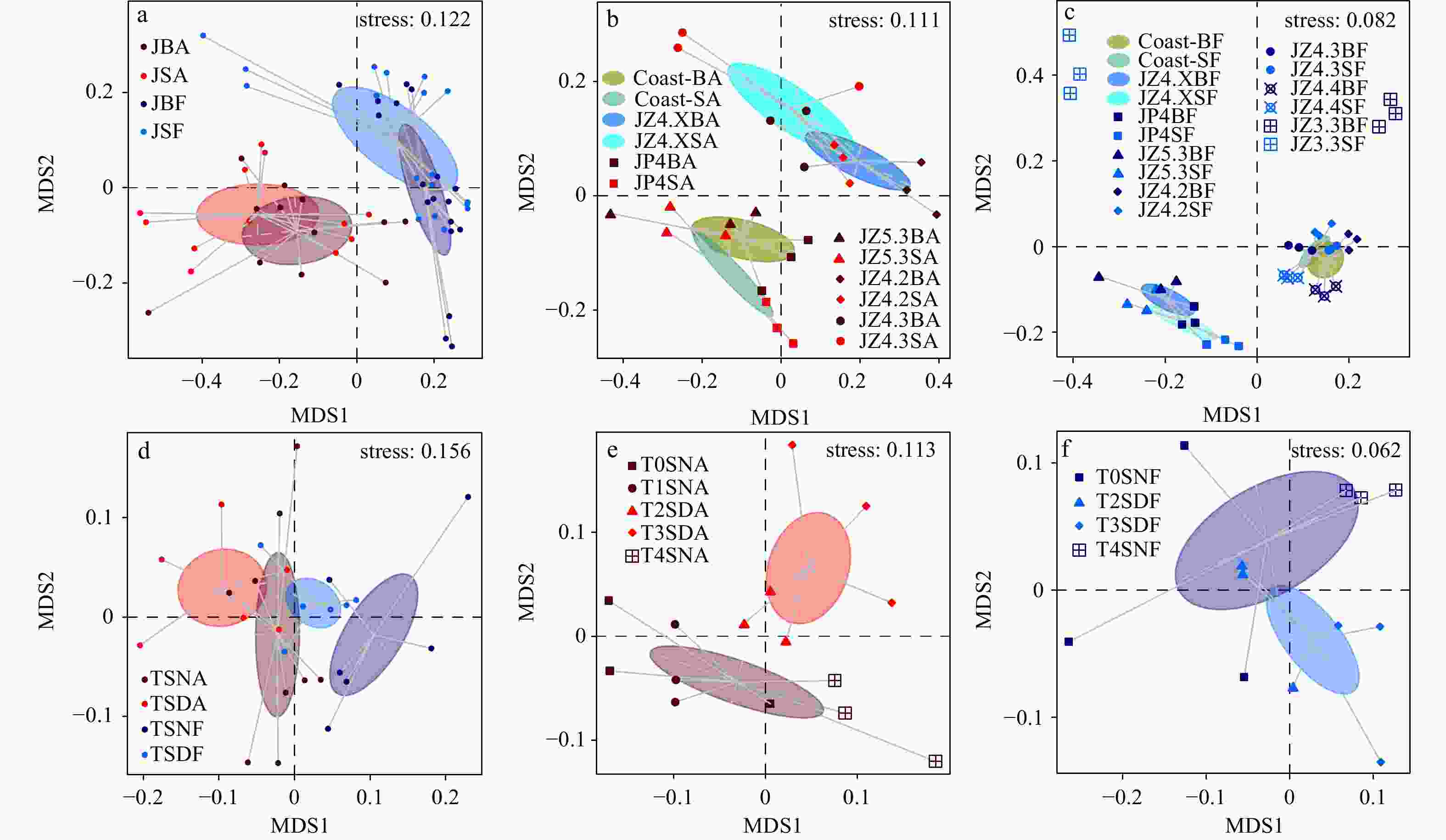
Figure 3. Non-metric multidimensional scaling (NMDS) ordination showed dissimilarities in the prokaryotic communities based on Bray-Curtis distances. Results from the first 2 ordination axes are given, with 80% confidence ellipses around the sample groups. a. NMDS plots of all spatial samples. b. The spatial samples in particle-attached (PA) fraction. c. The spatial samples in free-living (FL) fraction. d. NMDS plots of all diel samples. e. The diel samples in PA fraction. f. The diel samples in FL fraction. Prefix: J, January. Suffix: BA, bottom particle-attached prokaryotes; SA, surface particle-attached prokaryotes; BF, bottom free-living prokaryotes; SF, surface free-living prokaryotes; SNA, surface night particle-attached prokaryotes; SDA, surface day particle-attached prokaryotes; SNF, surface night free-living prokaryotes; SDF, surface day free-living prokaryotes (the results of ANOSIM are listed in Table S4).
Figure 4. Distance-based Redundancy Analyses (dbRDA) ordination plot represented the environmental variables that have influences (arrows) on the distribution of prokaryotic communities based on operationaltaxonomic unit (OTU) abundance. a. The spatial samples in particle-attached (PA) fraction. b. The spatial samples in free-living (FL) fraction. c. The diel samples in PA fraction. d. The diel samples in FL fraction. Prefix: J, January. Suffix: BA, bottom particle-attached prokaryotes; SA, surface particle-attached prokaryotes; BF, bottom free-living prokaryotes; SF, surface free-living prokaryotes; SNA, surface night particle-attached prokaryotes; SDA, surface day particle-attached prokaryotes; SNF, surface night free-living prokaryotes; SDF, surface day free-living prokaryotes (the significance of each environmental variable is listed in Table S5). T, temperature; S, salinity; Chl a, chlorophyll a; P. g, the abundance of P. globosa colony; ColD, collection depth;
${\rm{NO}}_3^- $ , nitrate nitrogen;${\rm{NO}}_2^- $ , nitrite nitrogen;${\rm{NH}}_4^+ $ , ammonia nitrogen, μmol/L;${\rm{SiO}}_3^{2-} $ , silicate. -
Alderkamp A C, Buma A G J, van Rijssel M. 2007. The carbohydrates of Phaeocystis and their degradation in the microbial food web. Biogeochemistry, 83(1–3): 99–118 Alderkamp A C, Nejstgaard J C, Verity P G, et al. 2006. Dynamics in carbohydrate composition of Phaeocystis pouchetii colonies during spring blooms in mesocosms. Journal of Sea Research, 55(3): 169–181. doi: 10.1016/j.seares.2005.10.005 Bauer A, Radziejewska T, Liang Kai, et al. 2013. Regional differences of hydrographical and sedimentological properties in the Beibu Gulf, South China Sea. Journal of Coastal Research, 66(2): 49–71 Bayer B, Vojvoda J, Reinthaler T, et al. 2019. Nitrosopumilus adriaticus sp. nov. and Nitrosopumilus piranensis sp. nov., two ammonia-oxidizing archaea from the Adriatic Sea and members of the class Nitrososphaeria. International Journal of Systematic and Evolutionary Microbiology, 69(7): 1892–1902. doi: 10.1099/ijsem.0.003360 Becquevort S, Rousseau V, Lancelot C. 1998. Major and comparable roles for free-living and attached bacteria in the degradation of Phaeocystis-derived organic matter in Belgian coastal waters of the North Sea. Aquatic Microbial Ecology, 14(1): 39–48 Buchan A, LeCleir G R, Gulvik C A, et al. 2014. Master recyclers: features and functions of bacteria associated with phytoplankton blooms. Nature Reviews Microbiology, 12(10): 686–698. doi: 10.1038/nrmicro3326 Celussi M, Paoli A, Aubry F B, et al. 2008. Diel microbial variations at a coastal northern Adriatic station affected by Po River outflows. Estuarine, Coastal and Shelf Science, 76(1): 36–44 Chen Zhenhua. 2013. Numerical simulation on seasonal variation of ocean circulation and its dynamic mechanism in the Beibu Gulf (in Chinese)[dissertation]. Qingdao: Ocean University of China Chen Zuozhi, Cai Wengui, Xu Shannan, et al. 2011. Risk assessment of coastal ecosystem in Beibu Gulf, Guangxi of South China. Chinese Journal of Applied Ecology (in Chinese), 22(11): 2977–2986 Clarke K R. 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology, 18(1): 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x Delmont T O, Hammar K M, Ducklow H W, et al. 2014. Phaeocystis antarctica blooms strongly influence bacterial community structures in the Amundsen Sea polynya. Frontiers in Microbiology, 5: 646 Fuhrman J A, Cram J A, Needham D M. 2015. Marine microbial community dynamics and their ecological interpretation. Nature Reviews Microbiology, 13(3): 133–146. doi: 10.1038/nrmicro3417 Fuhrman J A, Eppley R W, Hagstrom A, et al. 1985. Diel variations in bacterioplankton, phytoplankton, and related parameters in the southern California Bight. Marine Ecology Progress Series, 27(1–2): 9–20 Gao Jingsong, Shi Maochong, Chen Bo, et al. 2014. Responses of the circulation and water mass in the Beibu Gulf to the seasonal forcing regimes. Acta Oceanologica Sinica, 33(7): 1–11. doi: 10.1007/s13131-014-0506-6 García F C, Alonso-Sáez L, Morán X A G, et al. 2015. Seasonality in molecular and cytometric diversity of marine bacterioplankton: the re-shuffling of bacterial taxa by vertical mixing. Environmental Microbiology, 17(10): 4133–4142. doi: 10.1111/1462-2920.12984 Gasol J M, Doval M D, Pinhassi J, et al. 1998. Diel variations in bacterial heterotrophic activity and growth in the northwestern Mediterranean Sea. Marine Ecology Progress Series, 164: 107–124. doi: 10.3354/meps164107 Ghiglione J F, Mevel G, Pujo-Pay M, et al. 2007. Diel and seasonal variations in abundance, activity, and community structure of particle-attached and free-living bacteria in NW Mediterranean Sea. Microbial Ecology, 54(2): 217–231. doi: 10.1007/s00248-006-9189-7 Gilbert J A, Field D, Swift P, et al. 2010. The taxonomic and functional diversity of microbes at a temperate coastal site: a ‘Multi-Omic’ study of seasonal and diel temporal variation. PLoS ONE, 5(11): e15545. doi: 10.1371/journal.pone.0015545 Hahnke S, Sperling M, Langer T, et al. 2013. Distinct seasonal growth patterns of the bacterium Planktotalea frisia in the North Sea and specific interaction with phytoplankton algae. FEMS Microbiology Ecology, 86(2): 185–199. doi: 10.1111/1574-6941.12151 Hanson C A, Fuhrman J A, Horner-Devine M C, et al. 2012. Beyond biogeographic patterns: processes shaping the microbial landscape. Nature Reviews Microbiology, 10(7): 497–506. doi: 10.1038/nrmicro2795 He Cheng, Song Shuqun, Li Caiwen. 2019. The spatial-temperal distribution of Phaeocystis globosa colonies and related affecting factors in Guangxi Beibu Gulf. Oceanologia et Limnologia Sinica, 50(3): 630–643 He Cheng, Xu Sha, Kang Zhenjun, et al. 2021. Prokaryotic community composition and structure during Phaeocystis globosa blooms in the Beibu Gulf, China. Aquatic Microbial Ecology, 86: 137–151. doi: 10.3354/ame01962 Hernando-Morales V, Varela M M, Needham D M, et al. 2018. Vertical and seasonal patterns control bacterioplankton communities at two horizontally coherent coastal upwelling sites off Galicia (NW Spain). Microbial Ecology, 76(4): 866–884. doi: 10.1007/s00248-018-1179-z Hong Yiguo. 2013. Marine nitrogen cycle recorded by nitrogen and oxygen isotope fractionation of nitrate. Advances in Earth Science (in Chinese), 28(7): 751–764 Huang Changjiang, Dong Qiaoxiang, Zheng Lei. 1999. Taxonomic and ecological studies on a large scale Phaeocystis pouchetii bloom in the southeast coast of China during late 1997. Oceanologia et Limnologia Sinica (in Chinese), 30(6): 581–590 Janse I, Zwart G, van der Maarel M J E C, et al. 2000. Composition of the bacterial community degrading Phaeocystis mucopolysaccharides in enrichment cultures. Aquatic Microbial Ecology, 22(2): 119–133 Kruskal J B. 1964. Nonmetric multidimensional scaling: a numerical method. Psychometrika, 29(2): 115–129. doi: 10.1007/BF02289694 Kuipers B, van Noort G J, Vosjan J, et al. 2000. Diel periodicity of bacterioplankton in the euphotic zone of the subtropical Atlantic Ocean. Marine Ecology Progress Series, 201: 13–25. doi: 10.3354/meps201013 Lai Junxiang, Jiang Fajun, Ke Ke, et al. 2014. Nutrients distribution and trophic status assessment in the northern Beibu Gulf, China. Chinese Journal of Oceanology and Limnology, 32(5): 1128–1144. doi: 10.1007/s00343-014-3199-y Lau W W Y, Keil R G, Armbrust E V. 2007. Succession and diel transcriptional response of the glycolate-utilizing component of the bacterial community during a spring phytoplankton bloom. Applied and Environmental Microbiology, 73(8): 2440–2450. doi: 10.1128/AEM.01965-06 Li Nan, Zhao Huaxian, Jiang Gonglingxia, et al. 2020. Phylogenetic responses of marine free-living bacterial community to Phaeocystis globosa bloom in Beibu Gulf, China. Frontiers in Microbiology, 11: 1624. doi: 10.3389/fmicb.2020.01624 Lutz K A, Wang Wenqin, Zdepski A, et al. 2011. Isolation and analysis of high quality nuclear DNA with reduced organellar DNA for plant genome sequencing and resequencing. BMC Biotechnology, 11(1): 54. doi: 10.1186/1472-6750-11-54 Mari X, Rassoulzadegan F, Brussaard C P D, et al. 2005. Dynamics of transparent exopolymeric particles (TEP) production by Phaeocystis globosa under N- or P-limitation: a controlling factor of the retention/export balance. Harmful Algae, 4(5): 895–914. doi: 10.1016/j.hal.2004.12.014 Martiny J B H, Bohannan B J M, Brown J H, et al. 2006. Microbial biogeography: putting microorganisms on the map. Nature Reviews Microbiology, 4(2): 102–112. doi: 10.1038/nrmicro1341 McArdle B H, Anderson M J. 2001. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology, 82(1): 290–297. doi: 10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2 Nelson C E, Carlson C A, Ewart C S, et al. 2014. Community differentiation and population enrichment of Sargasso Sea bacterioplankton in the euphotic zone of a mesoscale mode-water eddy. Environmental Microbiology, 16(3): 871–887. doi: 10.1111/1462-2920.12241 Olapade O A. 2012. Diel fluctuations in the abundance and community diversity of coastal bacterioplankton assemblages over a tidal cycle. Microbial Ecology, 63(1): 96–102. doi: 10.1007/s00248-011-9940-6 Peperzak L, Colijn F, Vrieling E G, et al. 2000. Observations of flagellates in colonies of Phaeocystis globosa (Prymnesiophyceae); a hypothesis for their position in the life cycle. Journal of Plankton Research, 22(12): 2181–2203. doi: 10.1093/plankt/22.12.2181 Qi Yuzao, Xu Ning, Wang Yan, et al. 2002. Progress of studies on red tide in China —Studies on Phaeocystis giobosa red tide and its DMS (DMSP) production. China Basic Science (in Chinese), 4: 23–28 Rink B, Martens T, Fischer D, et al. 2008. Short-term dynamics of bacterial communities in a tidally affected coastal ecosystem. FEMS Microbiology Ecology, 66(2): 306–319. doi: 10.1111/j.1574-6941.2008.00573.x Rooney-Varga J N, Giewat M W, Savin M C, et al. 2005. Links between phytoplankton and bacterial community dynamics in a coastal marine environment. Microbial Ecology, 49(1): 163–175. doi: 10.1007/s00248-003-1057-0 Rousseau V, Becquevort S, Parent J Y, et al. 2000. Trophic efficiency of the planktonic food web in a coastal ecosystem dominated by Phaeocystis colonies. Journal of Sea Research, 43(3–4): 357–372 Salazar G, Cornejo-Castillo F M, Borrull E, et al. 2015. Particle-association lifestyle is a phylogenetically conserved trait in bathypelagic prokaryotes. Molecular Ecology, 24(22): 5692–5706. doi: 10.1111/mec.13419 Salter I, Galand P E, Fagervold S K, et al. 2015. Seasonal dynamics of active SAR11 ecotypes in the oligotrophic Northwest Mediterranean Sea. The ISME Journal, 9(2): 347–360. doi: 10.1038/ismej.2014.129 Sapp M, Wichels A, Wiltshire K H, et al. 2007. Bacterial community dynamics during the winter-spring transition in the North Sea. FEMS Microbiology Ecology, 59(3): 622–637. doi: 10.1111/j.1574-6941.2006.00238.x Schoemann V, Becquevort S, Stefels J, et al. 2005. Phaeocystis blooms in the global ocean and their controlling mechanisms: a review. Journal of Sea Research, 53(1–2): 43–66 Sheik A R, Brussaard C P D, Lavik G, et al. 2014. Responses of the coastal bacterial community to viral infection of the algae Phaeocystis globosa. The ISME Journal, 8(1): 212–225. doi: 10.1038/ismej.2013.135 Smith W O Jr, McGillicuddy D J Jr, Olson E B, et al. 2017. Mesoscale variability in intact and ghost colonies of Phaeocystis antarctica in the Ross Sea: Distribution and abundance. Journal of Marine Systems, 166: 97–107. doi: 10.1016/j.jmarsys.2016.05.007 Spilmont N, Denis L, Artigas L F, et al. 2009. Impact of the Phaeocystis globosa spring bloom on the intertidal benthic compartment in the eastern English Channel: a synthesis. Marine Pollution Bulletin, 58(1): 55–63. doi: 10.1016/j.marpolbul.2008.09.007 Strickland J D H, Parsons T R. 1972. A practical Handbook of Seawater Analysis. 2nd ed. Ottawa: Fisheries Research Board of Canada, 201–206 Takahashi S, Tomita J, Nishioka K, et al. 2014. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE, 9(8): e105592. doi: 10.1371/journal.pone.0105592 Todd J D, Curson A R J, Nikolaidou-Katsaraidou N, et al. 2010. Molecular dissection of bacterial acrylate catabolism-unexpected links with dimethylsulfoniopropionate catabolism and dimethyl sulfide production. Environmental Microbiology, 12(2): 327–343. doi: 10.1111/j.1462-2920.2009.02071.x van Rijssel M, Janse I, Noordkamp D J B, et al. 2000. An inventory of factors that affect polysaccharide production by Phaeocystis globosa. Journal of Sea Research, 43(3–4): 297–306 Wang Kai, Ye Xiansen, Chen Heping, et al. 2015. Bacterial biogeography in the coastal waters of northern Zhejiang, East China Sea is highly controlled by spatially structured environmental gradients. Environmental Microbiology, 17(10): 3898–3913. doi: 10.1111/1462-2920.12884 Winter C, Herndl G J, Weinbauer M G. 2004. Diel cycles in viral infection of bacterioplankton in the North Sea. Aquatic Microbial Ecology, 35(3): 207–216 Wöhlbrand L, Wemheuer B, Feenders C, et al. 2017. Complementary metaproteomic approaches to assess the bacterioplankton response toward a phytoplankton spring bloom in the southern North Sea. Frontiers in Microbiology, 8: 442 Xu Yixiao, Zhang Teng, Zhou Jin. 2019. Historical occurrence of algal blooms in the northern Beibu Gulf of China and implications for future trends. Frontiers in Microbiology, 10: 451. doi: 10.3389/fmicb.2019.00451 Yu Shuxian, Pang Yunlong, Wang Yinchu, et al. 2018. Distribution of bacterial communities along the spatial and environmental gradients from Bohai Sea to northern Yellow Sea. PeerJ, 6: e4272. doi: 10.7717/peerj.4272 Yu Zhiming, Song Xiuxian, Cao Xihua, et al. 2017. Mitigation of harmful algal blooms using modified clays: theory, mechanisms, and applications. Harmful Algae, 69: 48–64. doi: 10.1016/j.hal.2017.09.004 Zehr J P, Kudela R M. 2010. Nitrogen cycle of the open ocean: from genes to ecosystems. Annual Review of Marine Science, 3: 197–225 Zu Tingting. 2005. Analysis of the current and its mechanism in the Gulf of Beibu (in Chinese)[dissertation]. Qingdao: Ocean University of China -



 下载:
下载: