Morphological and histological changes in the brains of turbot (Scophthalmus maximus) with gonadal development
-
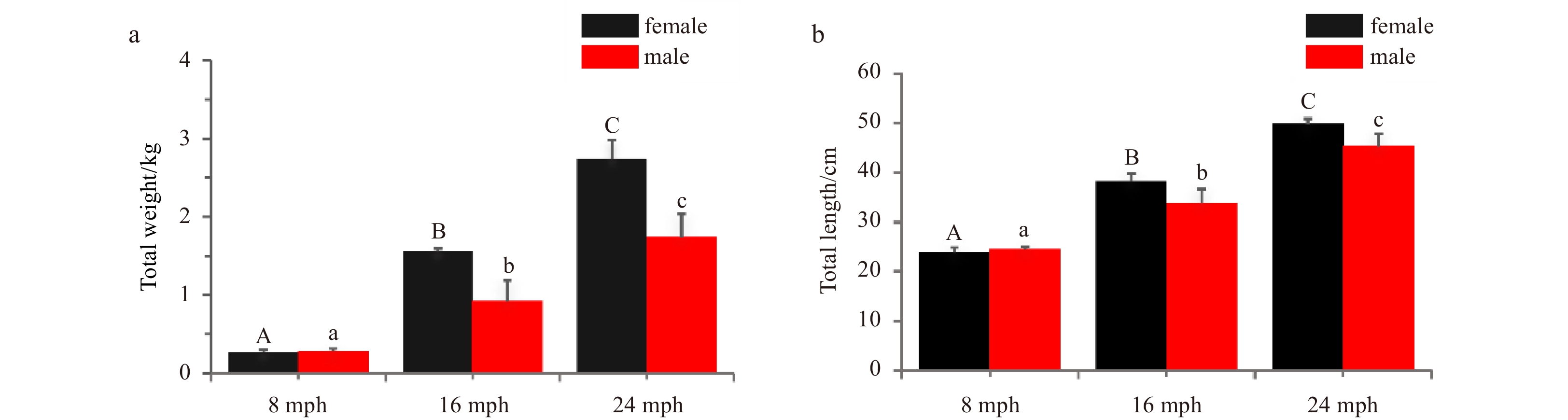
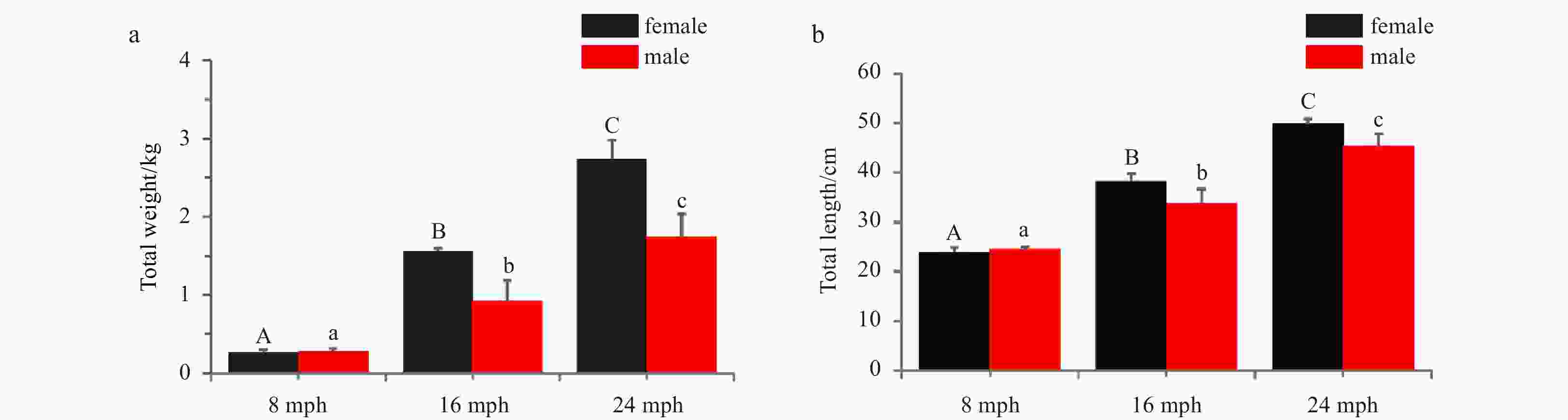
Abstract: The brain plays a critical role in controlling reproduction through the hypothalamus-pituitary-gonadal (HPG) axis in vertebrates. Turbot (Scophthalmus maximus) has become an economically important marine fish in Europe and North China. Previous research investigating turbot reproduction has focused on the role of the HPG axis in regulating egg and sperm production. However, the morphology and histology of the organs in the HPG axis have not been studied. In this study, we investigated the morphology and histology of brains in female and male turbot at different stages of gonadal development. The results showed that the brains of both female and male turbot were composed of seven parts that are typical of advanced teleosts: the telencephalon, diencephalon, cerebellum, hypothalamus, pituitary gland, myelencephalon, and olfactory bulbs. The telencephalon was well-developed and contained five distinct lobes, with the contiguous diencephalon at the caudal portion. The torus longitudinales and rostral torus semicircularis of the mesencephalon flattened along the dorsal surface, and the rostral corpus cerebellum was located in the dorsal portion. The actual total brain volume in mature males was significantly greater (p<0.05) than that of females with gonadal development. Notably, the pituitary volume in male turbot significantly increased (p<0.05) from immature to mature stage, but this difference did not occur in females. The data together illustrate a distinct sex difference in the turbot brain during gonadal development, providing insight into their HPG axes.
-
Key words:
- turbot /
- brain /
- pituitary /
- gonadal development /
- morphology /
- histology
-
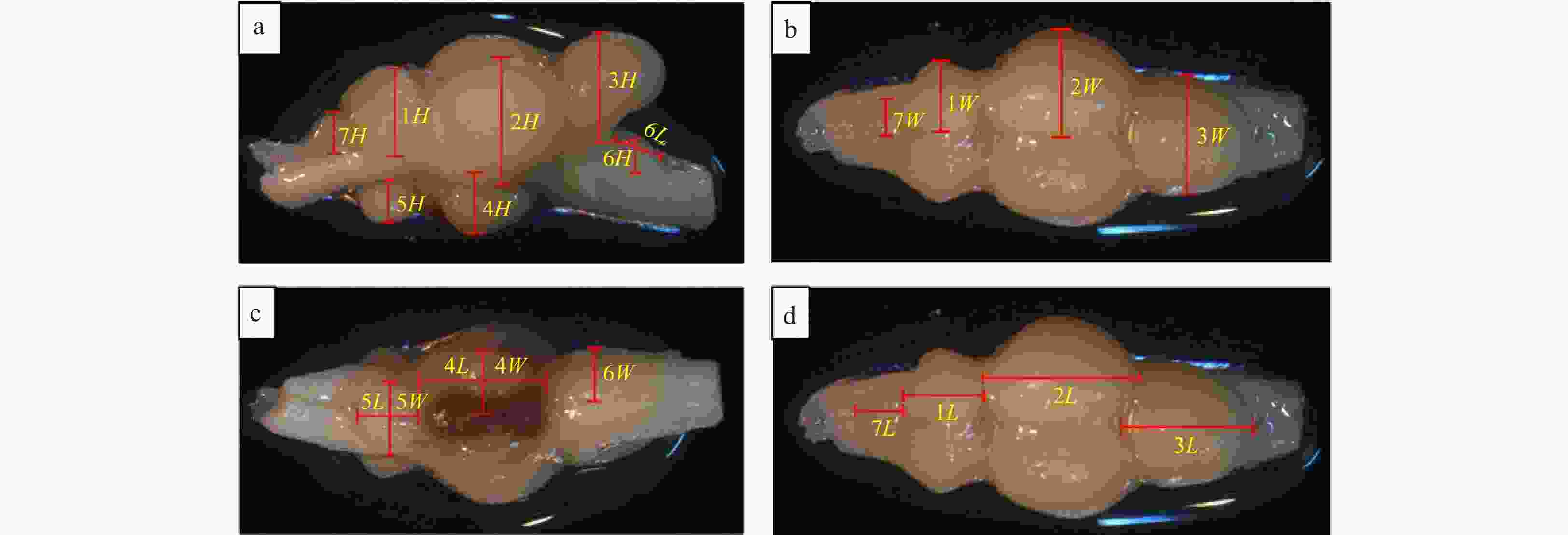
Figure 2. Illustration of the ellipsoid method to assess brain volumes of turbot. a. Lateral view; b. dorsal view; c. ventral view; d. dorsal view. The measurements used to determine the sizes of the brain structures are indicated. W represents width; H, height; L, length; 1, telencephalon; 2, diencephalon; 3, cerebellum; 4, hypothalamus and saccus vasculosus; 5, pituitary; 6, myelencephalon; 7, olfactory bulb.
Figure 4. Transverse sections of brain of turbot (I). a–d. Telencephalon, diencephalon, mesencephalon, and cerebellum. Tissue abbreviations: Ac represents anterior commissure; Vp, ventral posterior nucleus; Dc, dorsocentral nucleus of the telencephalon; Dd, dorsodorsal nucleus; Dl, dorsolateral nucleus of the telencephalon; Dm, dorsomedial nucleus of the telencephalon; DM, dorsal medial nucleus of the thalamus; Dp, dorsal posterior nucleus of the telencephalon; G, nucleus glomerulosus; Ha, habenula; III, oculomotor nerve; LL, lateral lemniscus; LR, lateral recess of the inferior lobe; MLF, medial longitudinal fasciculus; ND, nucleus diffusus of the inferior lobe; NPTec, pretectal nucleus; OB, olfactory bulb; PM, magnocellular nucleus; PP, preoptic nucleus; Vcb, valvula cerebelli; SV, saccus vasculosus; Tec, tectum of the mesencephalon; Tl, torus longitudinalis of the mesencephalon; Ts, torus semicircularis of the mesencephalon. Scale: 1 mm.
Figure 5. Transverse sections of brain of turbot (II). a–d. Cerebellum and myelencephalon. Tissue abbreviations: BC represents brachium conjunctivum; CC, crista cerebellaris; CCb, corpus division of the cerebellum; VIII, auditory/vestibular nerve complex; Tec, tectum of the mesencephalon; EG, eminentia granularis; LL, lateral lemniscus; MLF, medial longitudinal fasciculus; RF, reticular formation; Vm, motor nucleus of the trigeminal nerve; Ts, torus semicircularis of the mesencephalon. Scale: 1mm.
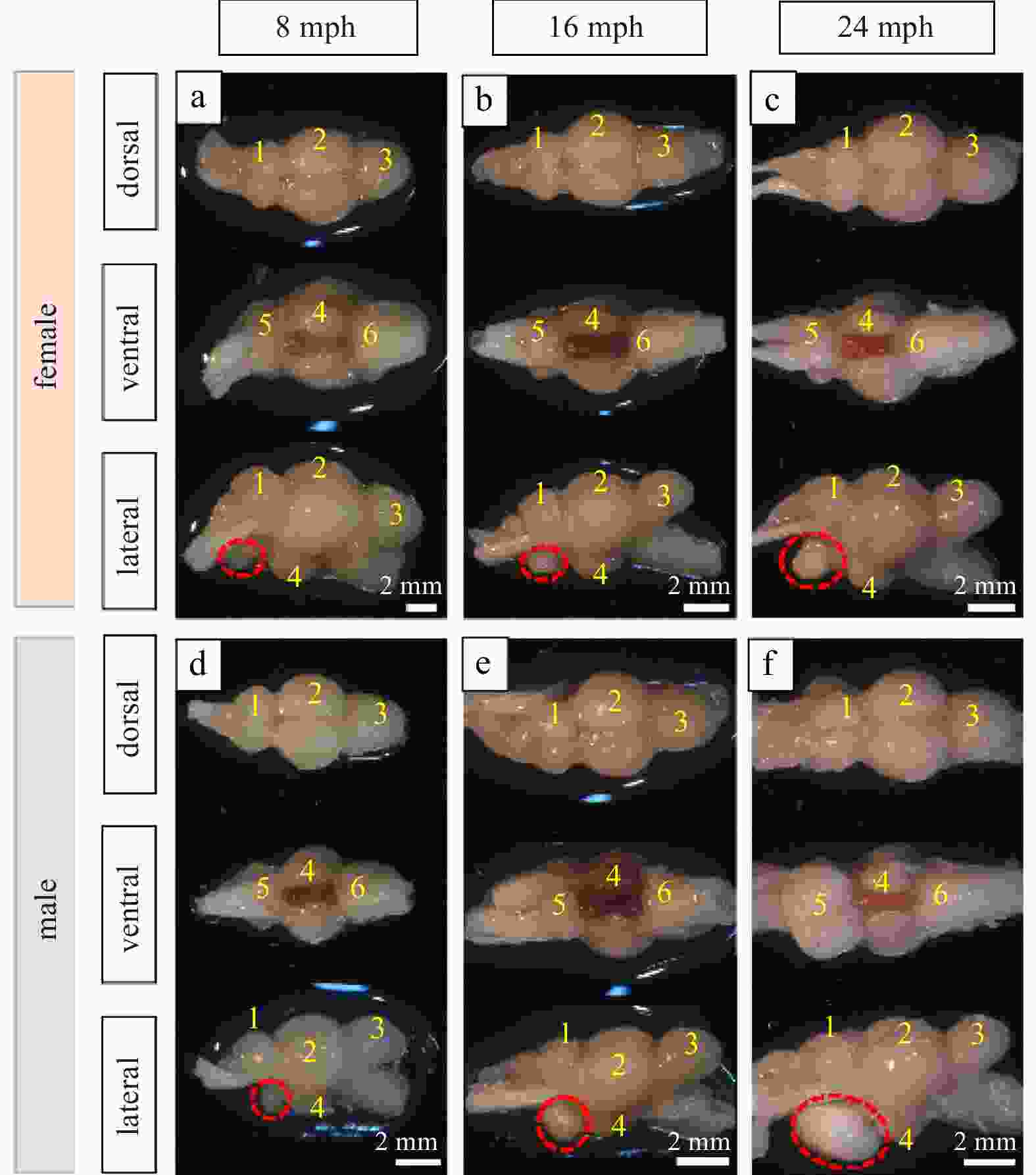
Figure 6. Morphology of turbot brain at 8 months post-hatching (mph) (a, d), 16 mph (b, e) and 24 mph (c, f). 1, telencephalon; 2, diencephalon; 3, cerebellum; 4, hypothalamus and saccus vasculosus; 5, pituitary; 6, myelencephalon. The pituitary gland is marked with a red dotted circle in the lateral view. Scale: 2 mm.
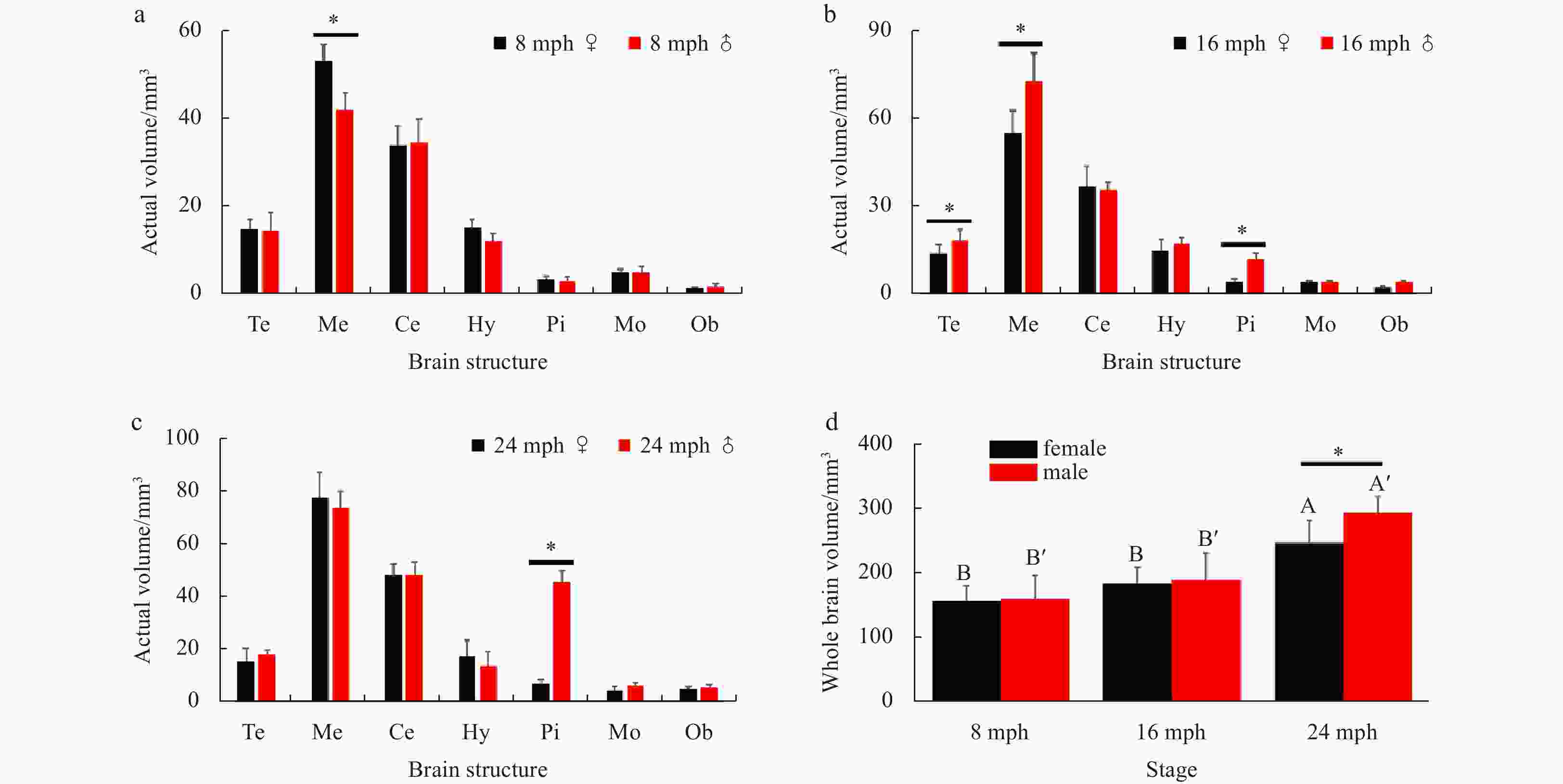
Figure 7. The actual brain structure volumes at different reproductive stages in both sexes. a. 8 months post-hatching (mph); b. 16 mph; c. 24 mph. d. the actual total brain volumes at different stages in both sexes. Abbreviations: Te, telencephalon; Me, mesencephalon; Ce, cerebellum; Hy, hypothalamus; Pi, pituitary glands; Mo, myelencephalon; Ob, olfactory bulb. Error bars represent mean±SD (N=15). Statistical significance (p<0.05) marked with A, B in female group, and A', B' in male group. Asterisk (*) indicates significant difference (p<0.05) between the sexes.
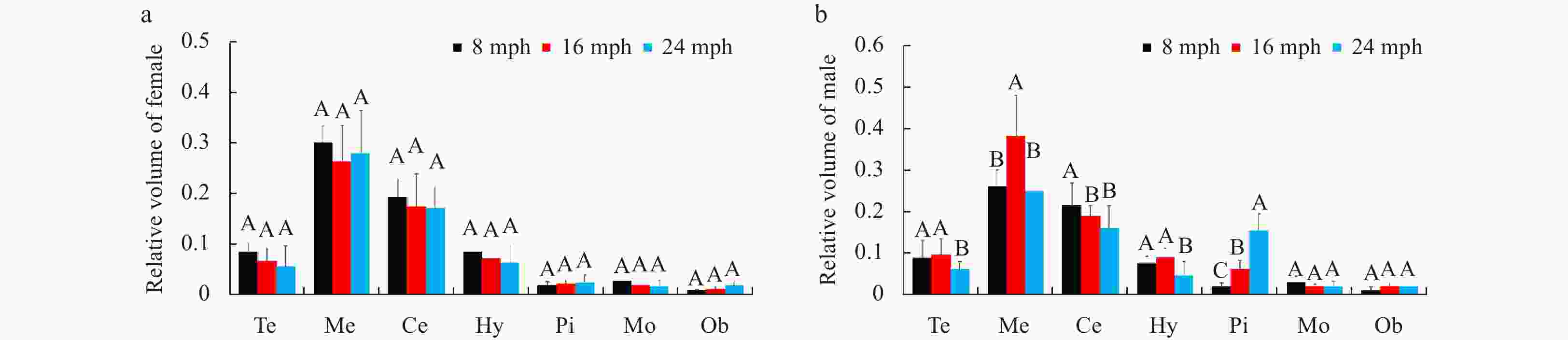
Figure 8. Comparison of relative brain structure volumes in female (a) and male (b) turbots at different stages. Abbreviations: Te, telencephalon; Me, mesencephalon; Ce, cerebellum; Hy, hypothalamus; Pi, pituitary glands; Mo, myelencephalon; Ob, olfactory bulb. Error bars represent mean±SD (N=15). Different letters represent significant differences among the stages (p<0.05).
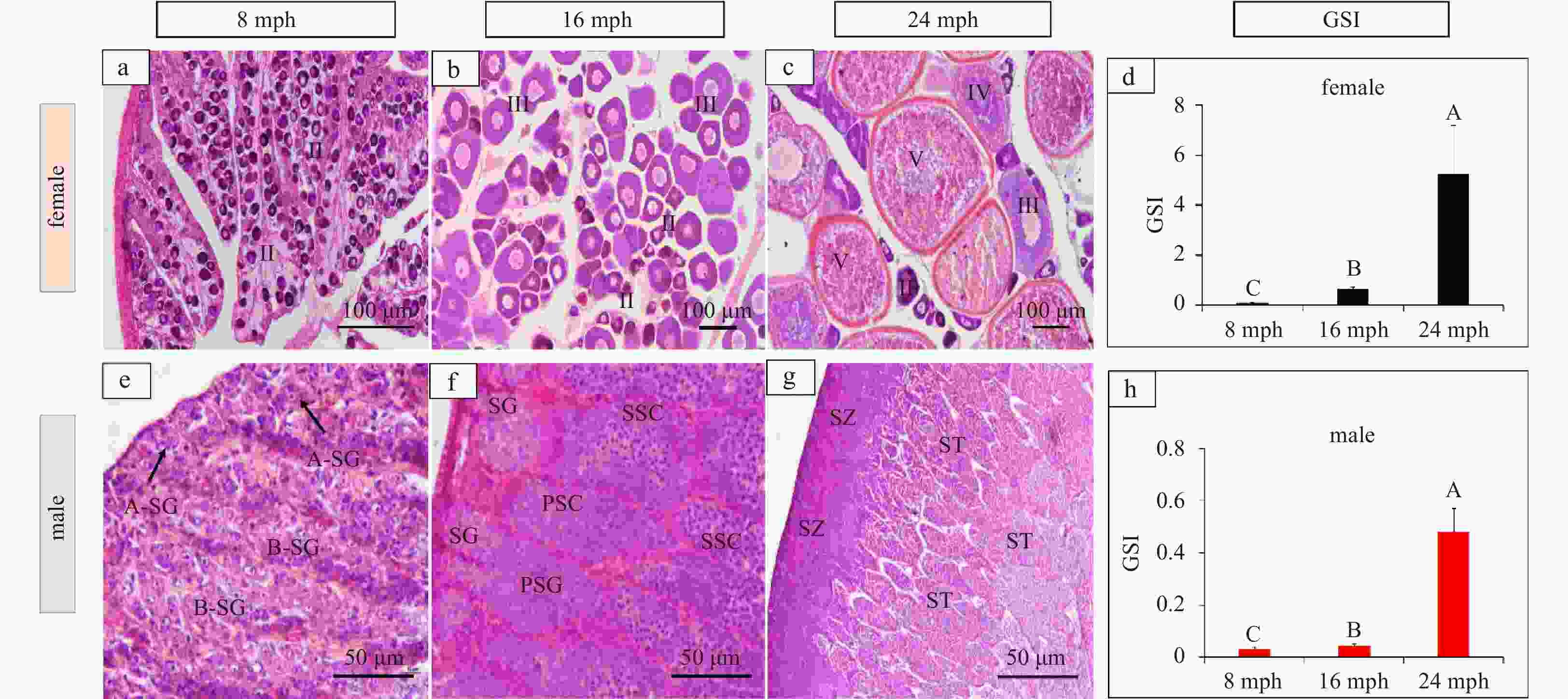
Figure 9. Histology of ovaries and testes at 8 months post-hatching (mph) (a, e), 16 mph (b, f), and 24 mph (c, g). d. Genadosomatic Index (GSI) of females; h. GSI of males. II, primary vitellogenic oocytes; III, IV, large growth of vitellogenic oocytes; V, mature oocytes. Abbreviations: A-SG, A type-spermatogonia; B-SG, B type-spermatogonia; PSC, primary spermatocytes; SSC, secondary spermatocytes; ST, spermatids; SZ, spermatozoa. Scale: a–c, 100 μm; e–g, 50 μm. Error bars represent mean±SD (N=3). Different letters represent significant differences among stages (p<0.05).
-
Acevedo-Rodriguez A, Kauffman A S, Cherrington B D, et al. 2018. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. Journal of Neuroendocrinology, 30(10): e12590 Alvariño J M R, Rebollar P G, Olmedo M, et al. 2001. Effects of melatonin implants on reproduction and growth of turbot broodstock. Aquaculture International, 9(6): 477–487. doi: 10.1023/A:1020590111031 Bauchot R, Ridet J M, Bauchot M L. 1989. The brain organization of butterflyfishes. Environmental Biology of Fishes, 25(1): 205 Bizzarri C, Cappa M. 2020. Ontogeny of hypothalamus-pituitary gonadal axis and minipuberty: an ongoing debate?. Frontiers in Endocrinology, 11: 187. Borella M I, Chehade C, Costa F G, et al. 2019. The brain-pituitary-gonad axis and the gametogenesis. In: Baldisserotto B, Urbinati E C, Cyrino J E P, eds. Biology and Physiology of Freshwater Neotropical Fish. Amsterdam: Academic Press, 315–341 Borella M I, Venturieri R, Mancera J M. 2009. Immunocytochemical identification of adenohypophyseal cells in the pirarucu (Arapaima gigas), an Amazonian basal teleost. Fish Physiology and Biochemistry, 35(1): 3–16. doi: 10.1007/s10695-008-9254-x Briñón J G, Médina M, Arévalo R, et al. 1993. Volumetric analysis of the telencephalon and tectum during metamorphosis in a flatfish, the turbot Scophthalmus maximus. Brain, Behavior and Evolution, 41(1): 1–5 Broglio C, Rodríguez F, Salas C. 2003. Spatial cognition and its neural basis in teleost fishes. Fish and Fisheries, 4(3): 247–255. doi: 10.1046/j.1467-2979.2003.00128.x Burns J G, Rodd F H. 2008. Hastiness, brain size and predation regime affect the performance of wild guppies in a spatial memory task. Animal Behaviour, 76(3): 911–922. doi: 10.1016/j.anbehav.2008.02.017 Demski L S. 2003. In a fish’s mind’s eye: the visual pallium of teleosts. In: Collin S P, Marshall N J, eds. Sensory Processing in Aquatic Environments. New York: Springer, 404–419 Dwyer A A, Quinton R. 2019. Anatomy and physiology of the hypothalamic-pituitary-gonadal (HPG) axis. In: Llahana S, Follin C, Yedinak C, et al., eds. Advanced Practice in Endocrinology Nursing. Cham: Springer, 839–852 Eastman J T, Lannoo M J. 2008. Brain and sense organ anatomy and histology of the Falkland Islands mullet, Eleginops maclovinus (Eleginopidae), the sister group of the Antarctic notothenioid fishes (Perciformes: Notothenioidei). Journal of Morphology, 269(1): 84–103. doi: 10.1002/jmor.10571 Finger T E. 1988. Organization of chemosensory systems within the brains of bony fishes. In: Atema J, Fay R R, Popper A N, et al., eds. Sensory Biology of Aquatic Animals. New York: Springer, 339–363 Gonda A, Herczeg G, Merilä J. 2009. Adaptive brain size divergence in nine-spined sticklebacks (Pungitius pungitius)?. Journal of Evolutionary Biology, 22(8): 1721–1726 Gonzalez-Voyer A, Winberg S, Kolm N. 2009a. Brain structure evolution in a basal vertebrate clade: evidence from phylogenetic comparative analysis of cichlid fishes. BMC Evolutionary Biology, 9(1): 238. doi: 10.1186/1471-2148-9-238 Gonzalez-Voyer A, Winberg S, Kolm N. 2009b. Social fishes and single mothers: brain evolution in African cichlids. Proceedings of the Royal Society B: Biological Sciences, 276(1654): 161–167. doi: 10.1098/rspb.2008.0979 Imsland A K, Dragsnes M, Stefansson S O. 2003. Exposure to continuous light inhibits maturation in turbot (Scophthalmus maximus). Aquaculture, 219(1–4): 911–919 Ishikawa Y, Yoshimoto M, Yamamoto N, et al. 1999. Different brain morphologies from different genotypes in a single teleost species, the Medaka (Oryzias latipes). Brain, Behavior and Evolution, 53(1): 2–9 Ito H, Ishikawa Y, Yoshimoto M, et al. 2007. Diversity of brain morphology in teleosts: brain and ecological niche. Brain, Behavior and Evolution, 69(2): 76–86 Kolm N, Gonzalez-Voyer A, Brelin D, et al. 2009. Evidence for small scale variation in the vertebrate brain: mating strategy and sex affect brain size and structure in wild brown trout (Salmo trutta). Journal of Evolutionary Biology, 22(12): 2524–2531. doi: 10.1111/j.1420-9101.2009.01875.x Kotrschal K, Krautgartner W D, Adam H. 1983. Crown cells in the diencephalon of Acipenser ruthenus (Acipenseridae, Chondrostei). Journal für Hirnforschung, 24(6): 655–657 Kotrschal K, van Staaden M J, Huber R. 1998. Fish brains: evolution and anvironmental relationships. Reviews in Fish Biology and Fisheries, 8(4): 373–408. doi: 10.1023/A:1008839605380 Lin Fan, Xu Shihong, Ma Daoyuan, et al. 2012. Germ line specific expression of a vasa homologue gene in turbot (Scophthalmus maximus): evidence for vasa localization at cleavage furrows in euteleostei. Molecular Reproduction and Development, 79(11): 803–813. doi: 10.1002/mrd.22120 Lin Fan, Zhao Chunyan, Xu Shihong, et al. 2013. Germline-specific and sexually dimorphic expression of a dead end gene homologue in turbot (Scophthalmus maximus). Theriogenology, 80(6): 665–672. doi: 10.1016/j.theriogenology.2013.06.016 Liu Yifan, Liu Qinghua, Xu Shihong, et al. 2021. A deep insight of spermatogenesis and hormone levels of aqua-cultured turbot (Scophthalmus maximus). Frontiers in Marine Science, 7: 592880. doi: 10.3389/fmars.2020.592880 Loveland J L, Giraldo-Deck L M, Lank D B, et al. 2021. Functional differences in the hypothalamic-pituitary-gonadal axis are associated with alternative reproductive tactics based on an inversion polymorphism. Hormones and Behavior, 127: 104877. doi: 10.1016/j.yhbeh.2020.104877 MacManes M D, Austin S H, Lang A S, et al. 2017. Widespread patterns of sexually dimorphic gene expression in an avian hypothalamic–pituitary–gonadal (HPG) axis. Scientific Reports, 7: 45125. doi: 10.1038/srep45125 Maharajan K, Muthulakshmi S, Karthik C, et al. 2020. Pyriproxyfen induced impairment of reproductive endocrine homeostasis and gonadal histopathology in zebrafish (Danio rerio) by altered expression of hypothalamus-pituitary-gonadal (HPG) axis genes. Science of the Total Environment, 735: 139496. doi: 10.1016/j.scitotenv.2020.139496 Meethal S V, Atwood C S. 2005. The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cellular and Molecular Life Sciences, 62(3): 257–270. doi: 10.1007/s00018-004-4381-3 Miwa S, Inui Y. 1987. Histological changes in the pituitary-thyroid axis during spontaneous and artificially-induced metamorphosis of larvae of the flounder Paralichthys olivaceus. Cell and Tissue Research, 249(1): 117–123. doi: 10.1007/BF00215425 Northcutt R G. 2006. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. Journal of Comparative Neurology, 494(6): 903–943. doi: 10.1002/cne.20853 Pitnick S, Jones K E, Wilkinson G S. 2006. Mating system and brain size in bats. Proceedings of the Royal Society B: Biological Sciences, 273(1587): 719–724. doi: 10.1098/rspb.2005.3367 Pollen A A, Dobberfuhl A P, Scace J, et al. 2007. Environmental complexity and social organization sculpt the brain in Lake Tanganyikan cichlid fish. Brain, Behavior and Evolution, 70(1): 21–39 Rao P D P, Finger T E. 1984. Asymmetry of the olfactory system in the brain of the winter flounder, Pseudopleuronectes americanus. Journal of Comparative Neurology, 225(4): 492–510. doi: 10.1002/cne.902250403 Safi K, Dechmann D K N. 2005. Adaptation of brain regions to habitat complexity: a comparative analysis in bats (Chiroptera). Proceedings of the Royal Society B: Biological Sciences, 272(1559): 179–186. doi: 10.1098/rspb.2004.2924 Stoss J H, Røer J E. 2020. Controlled reproduction in turbot (Scopthalmus maximus). In: Reinertsen H, Dahle L A, Jørgensen L, et al., eds. Fish Farming Technology. London: CRC Press Striedter G F, Northcutt R G. 2006. Head size constrains forebrain development and evolution in ray-finned fishes. Evolution and Development, 8: 215-222 Ullmann J F P, Cowin G, Collin S P. 2010. Quantitative assessment of brain volumes in fish: comparison of methodologies. Brain, Behavior and Evolution, 76(3–4): 261–270 White G E, Brown C. 2015a. Microhabitat use affects brain size and structure in intertidal gobies. Brain, Behavior and Evolution, 85(2): 107–116 White G E, Brown C. 2015b. Variation in brain morphology of intertidal gobies: a comparison of methodologies used to quantitatively assess brain volumes in fish. Brain, Behavior and Evolution, 85(4): 245–256 Xu Wengang, Manabe S, Mushirobira Y, et al. 2020. Changes in expression of reproduction-related hormones in the brain and pituitary during early ovarian differentiation and development in the red spotted grouper Epinephelus akaara, with emphasis on FSHβ and LHβ. Aquaculture, 514: 734497. doi: 10.1016/j.aquaculture.2019.734497 Xue Rui, Wang Xueying, Xu Shihong, et al. 2018. Expression profile and localization of vitellogenin mRNA and protein during ovarian development in turbot (Scophthalmus maximus). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 226: 53–63. doi: 10.1016/j.cbpb.2018.08.002 Zhao Chunyan, Liu Qinghua, Xu Shihong, et al. 2018a. Identification of type A spermatogonia in turbot (Scophthalmus maximus) using a new cell-surface marker of Lymphocyte antigen 75 (ly75/CD205). Theriogenology, 113: 137–145. doi: 10.1016/j.theriogenology.2017.12.016 Zhao Chunyan, Xu Shihong, Feng Chengcheng, et al. 2018b. Characterization and differential expression of three GnRH forms during reproductive development in cultured turbot Schophthalmus maximus. Journal of Oceanology and Limnology, 36(4): 1360–1373. doi: 10.1007/s00343-018-7068-y Zhao Chunyan, Xu Shihong, Liu Yifan, et al. 2017. Gonadogenesis analysis and sex differentiation in cultured turbot (Scophthalmus maximus). Fish Physiology and Biochemistry, 43(1): 265–278. doi: 10.1007/s10695-016-0284-5 Zhou Li, Wang Xueying, Liu Qinghua, et al. 2019. Visualization of turbot (Scophthalmus maximus) primordial germ cells in vivo using fluorescent protein mediated by the 3′ untranslated region of nanos3 or vasa gene. Marine Biotechnology, 21(5): 671–682. doi: 10.1007/s10126-019-09911-z -



 下载:
下载: