A simple guideline to apply excitation-emission matrix spectroscopy (EEMs) for the characterization of dissolved organic matter (DOM) in anoxic marine sediments
-
Abstract: Marine sediments represent a major carbon reservoir on Earth. Dissolved organic matter (DOM) in pore waters accumulates products and intermediates of carbon cycling in sediments. The application of excitation-emission matrix spectroscopy (EEMs) in the analysis of subseafloor DOM samples is largely unexplored due to the redox-sensitive matrix of anoxic pore water. Therefore, this study aims to investigate the interference caused by the matrix on EEMs and propose a guideline to prepare pore water samples from anoxic marine sediments. The parameters determined by fluorescence spectra include 3D-index derived from EEMs after parallel factor analysis (PARAFAC), fluorescence index (FI) (contribution of terrigenous DOM), biological index (BIX) and humification index (HIX) derived from 2D emission spectra. First, we investigated the impacts of extensively-presented ions as typical electron acceptors, which are utilized by anaerobic microbes and stratified in marine sediments: Fe(II), Fe(III), Mn(II) and sulfide in anoxic pore water resulted in biases of fluorescent signals. We proposed threshold concentrations of these ions when the interference on EEMs occurred. Effective removal of sulfide from sulfide-rich samples could be achieved by flushing with N2 for 2 min. Second, the tests based on DOM standard were further verified using pristine samples from marine sediments. There was a significant change in the fluorescence spectra of DOM in anoxic sediments from the Rhône Delta. This study demonstrated that the change was caused by oxidation of the matrix rather than the intrinsic alteration of DOM. It was confirmed by extracted DOM via both EEMs analysis and Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS). Slight oxidation of sulfur-containing compounds (e.g., sulfhydryl) and polyphenol-like compounds occurred. Finally, a sample preparation sequence is proposed for pore water from anoxic sediments. This method enables measurement with small volumes of the sample (e.g., 50 µL in this study) and ensures reliable data without the interference of the redox-sensitive matrix. This study provides access to the rapid analysis of DOM composition in marine sediments and can potentially open a window into examining the carbon cycling of the marine deep biosphere.
-
Key words:
- marine subsurface sediment /
- EEMs /
- PARAFAC /
- FT-ICR-MS /
- anaerobic pore water
-
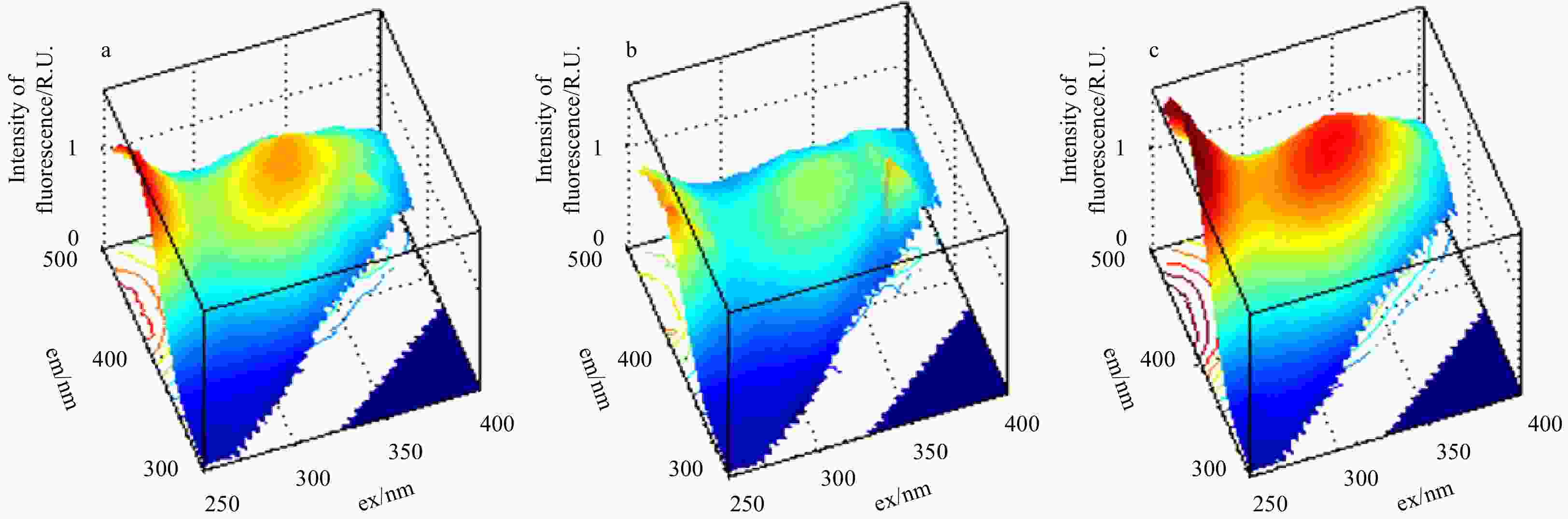
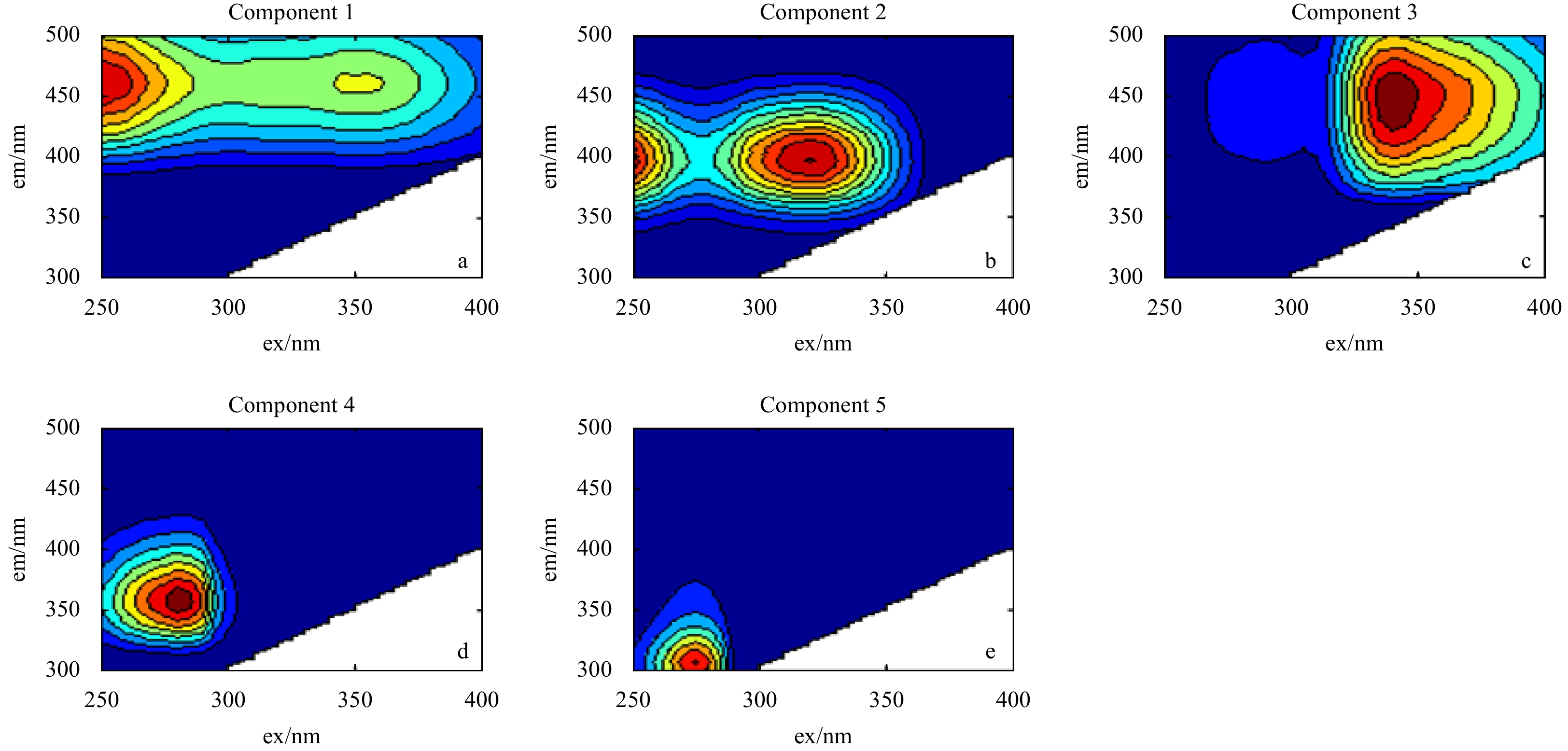
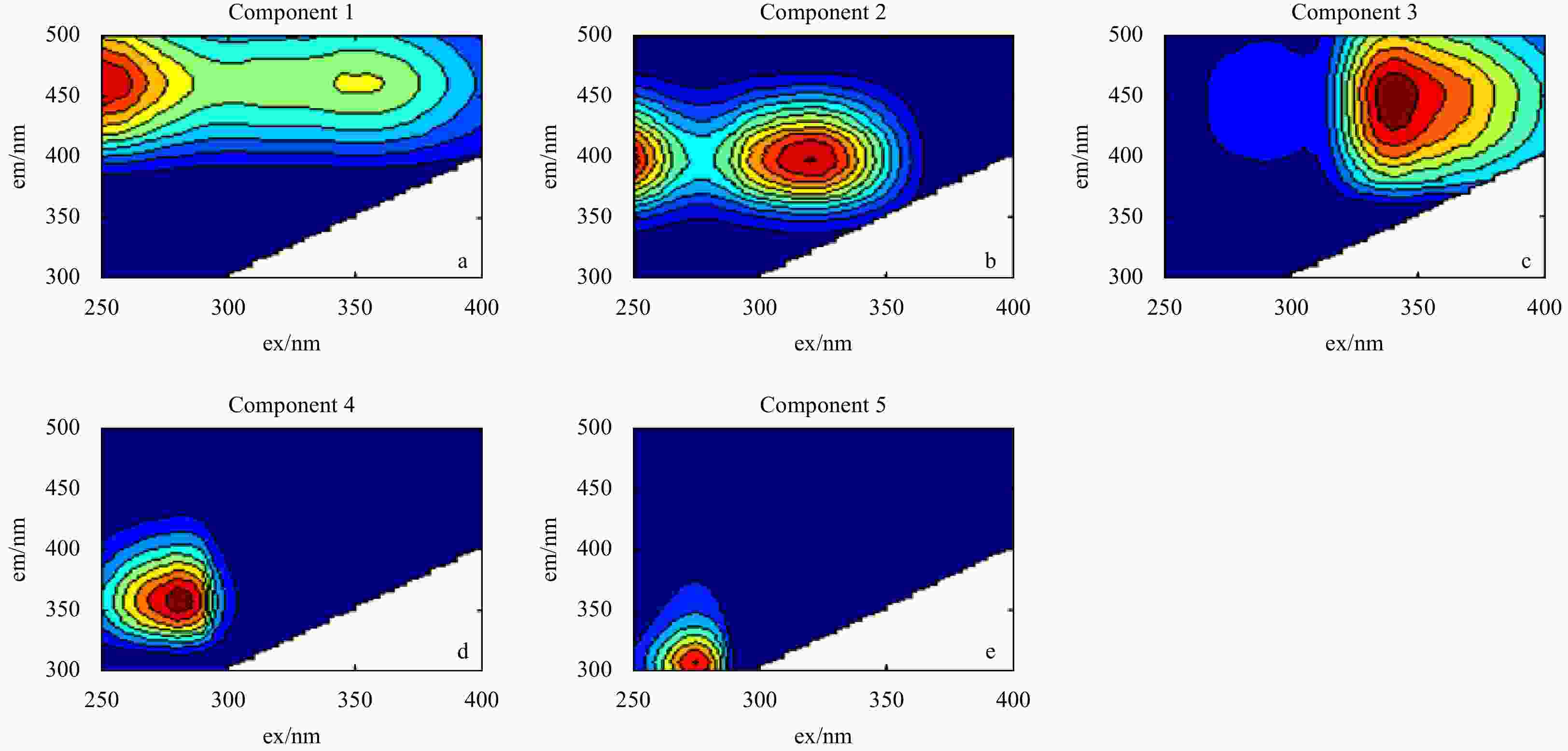
Figure 1. Excitation-emission matrix spectroscopy (EEMs) components identified by parallel factor analysis (PARAFAC). Components 1, 2, 3, 4 and 5 represent Peaks A(C), M, C, T and B, respectively. More information is shown in Table S1. The em and ex represent emission and excitation wavelength, respectively.
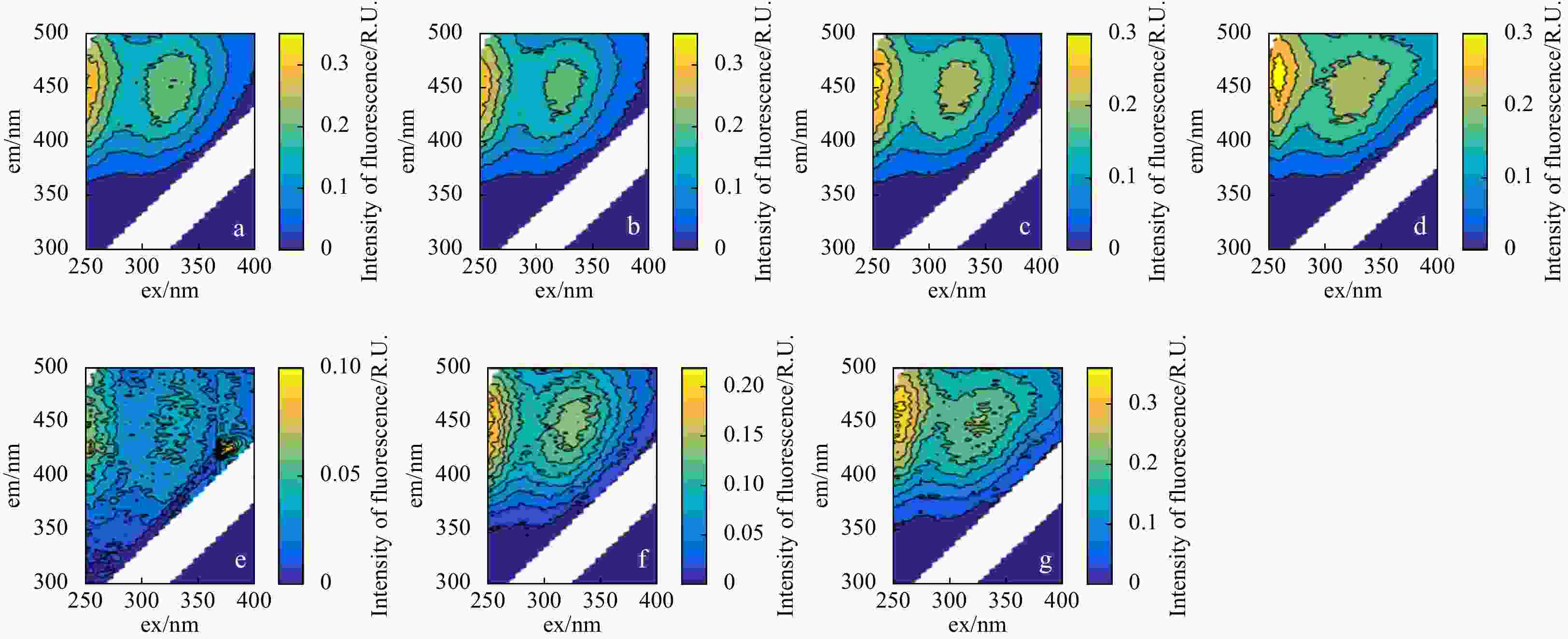
Figure 3. Effect of redox-sensitive ions on humic-like peaks. a. Excitation-emission matrix spectroscopy (EEMs) spectra of original Suwanee River Fulvic Acid Standard (SRFA) samples; b–d. EEMs spectra of SRFA samples with addition of Fe(II), Mn(II), S2– at concentrations of 0.06 mmol/L, 0.06 mmol/L, 0.3 mmol/L, respectively; e–g. EEMs of SRFA samples with addition of Fe(II), Mn(II), S2– at concentrations of 0.6 mmol/L, 0.6 mmol/L, 1 mmol/L, respectively. The em and ex represent emission and excitation wavelength, respectively.
Figure 4. Effect of redox-sensitive ions on protein-like peaks. a. Original excitation-emission matrix spectroscopy (EEMs) of yeast extract (YE); b–d. EEMs of YE samples with addition of Fe(III), Mn(II), S2– at concentrations of 0.2 mmol/L, 0.6 mmol/L, 1 mmol/L, respectively. The em and ex represent emission and excitation wavelength, respectively.
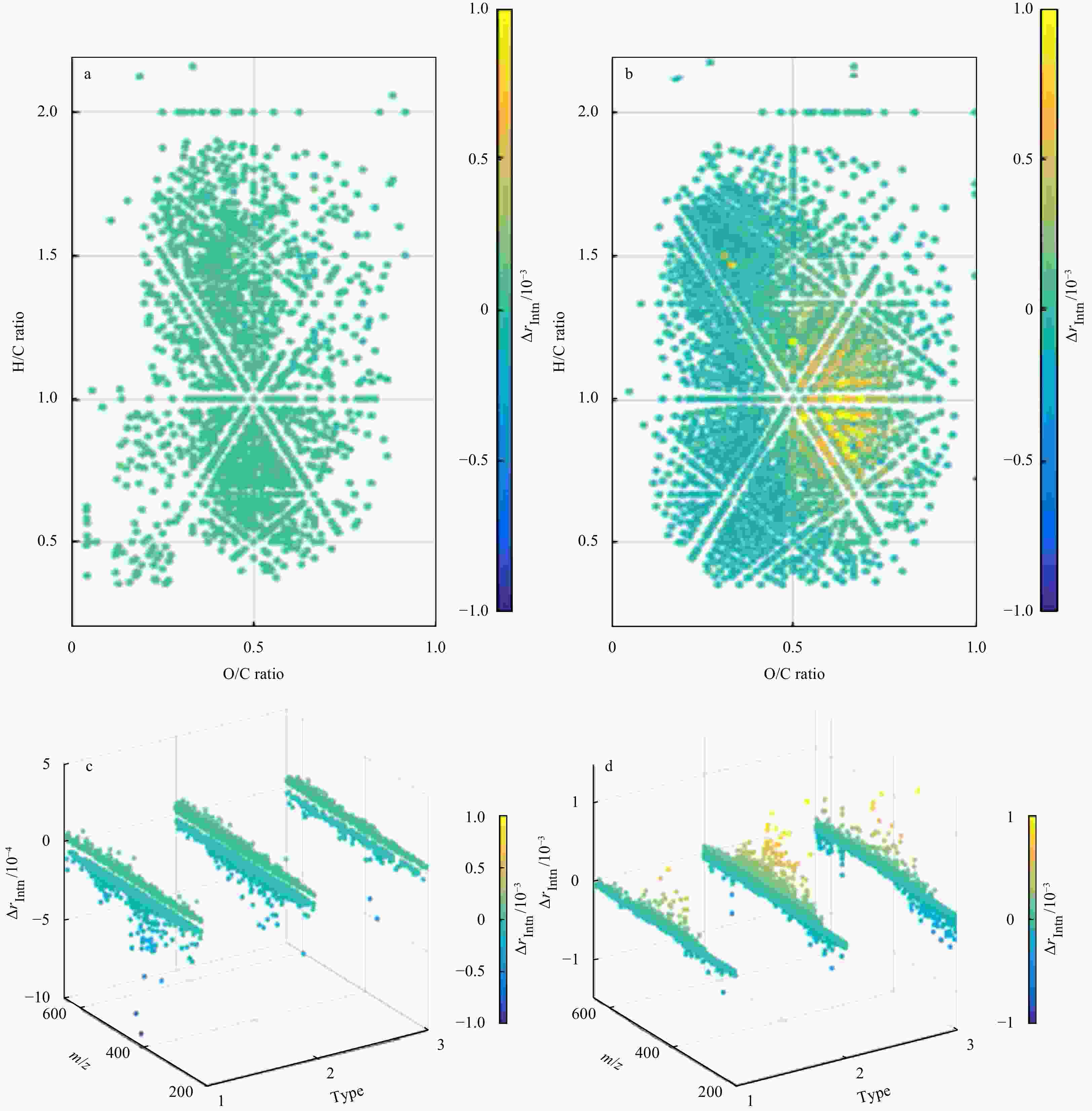
Figure 5. Effect of two-month O2 exposure on pore water dissolved organic matter (DOM) characterized by FT-ICR-MS. Change of DOM sample from the North Sea (a) and Rhône Delta (b) in van Krevelen diagram; change of O/C ratio in DOM sample from the North Sea (c) and Rhône Delta (d). Type 1: aliphatic compounds, AI<0; Type 2: highly unsaturated compounds, 0.5≥AI≥0; Type 3: aromatic compounds (including condense aromatic compounds), AI>0.5. The peak magnitude shown in the figure is relative intensity normalized to the sum of all peaks, i.e., rIntn= IPeak/∑IallPeaks. The color represents the difference of rIntn after air exposure (
$\Delta r_{\rm{Intn}} $ ), i.e., rIntn-end−rIntn-start. rIntn>0: relative abundance of formulae increases after air exposure. The y-axis in c and d shows the difference of rIntn at a narrower range. The definition of AI refers to Eq. (3).Table 1. Summary of main subseries experiments and controls
Experiment Sample, matrix or treatment Purpose of tests Incubation of sediments North Sea sample (20℃) sulfide-rich samples North Sea sample (85℃) samples enriched in protein-like compounds and humic-like compounds Rhône Delta sample (20℃) metal-ion-rich samples Matrix effects S2– impacts of anions involved in anoxic mineralization Mn(II), Fe(II), Fe(III) impacts of metal ions involved in anoxic mineralization matrix-removed DOM method to avoid matrix effects Storage effects DOM extracts, O2 exposure oxidation of DOM pristine samples, Rhône impacts of matrix oxidation during storage under O2 and method to avoid storage effects pristine samples, North Sea impacts of matrix oxidation during storage under O2 and method to avoid storage effects Note: The pristine sample refers to original pore water without solid phase extraction (SPE); the dissolved organic matter (DOM) extracts refer to purified sample without inorganic matrix after SPE. Table 2. Change of fluorescent signal along with metal ions, sulfide and O2 exposure. Acceptable ranges of concentrations are listed
Acceptable range Index Fe(III)
0–0.007 mmol/LFe(II)
0–0.06 mmol/LMn(II)
0–0.06 mmol/LNa2S
(N2-flushed)Extracted DOM after O2 exposure
2 monthsBias of 2D index FI ↑ NS NS ↑ ↓ HIX ↓ ↓ ↓ ↓ ↑ BIX ↑ NS NS ↑ NS Bias of 3D index protein-like peaks ↓ ↓ ↓ ↑ / humic-like peaks ↓ ↓ ↓ NS / AC/M ↓ ↓ ↓ ↑ NS Note: The relative changes below 5% were defined as NS, i.e., no significant impact. For 2D index with variation coefficient ranging from 5% to 15%, P<0.05 in ANOVA were defined as NS. Results of ANOVA are presented in Table S3. ↑ and ↓ represent increase and decrease of the parameters with higher concentrations of added ions or O2 exposure. FI: fluorescence index; BIX: biological index; HIX: humification index. AC/M: peak height ratio of Peak AC to Peak M. Table 3. Comparisons of dissolved organic matter (DOM) fluorescence spectra before and after solid phase extraction (SPE) by pre-cleaned Bond Elut-PPL cartridge
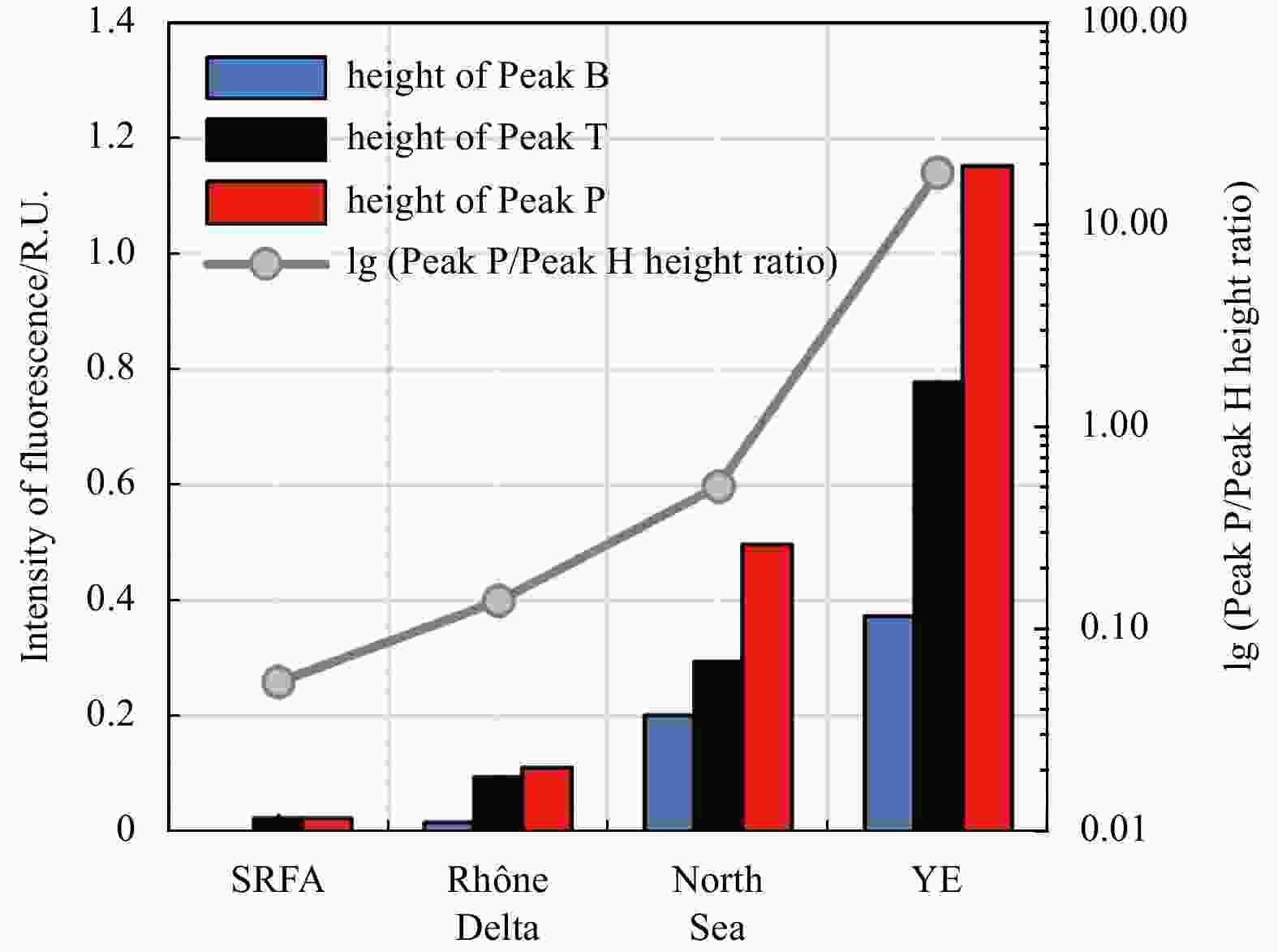
Sample P/H AC/M FI BIX HIX Before SPE-pristine sample 0.5 1.2 1.5 0.9 4.3 After SPE-DOM extract 0.2 1.5 1.6 0.6 8.1 After SPE-residue liquid 1.5 0.6 1.8 1.2 0.5 Standard deviation-pristine sample 0.01 0.01 0.02 0.1 0.2 Note: A 20-mL liquid sample was used for SPE. In-house-prepared samples from the North Sea sediments after incubation were tested since the pristine sample contains both substantial protein-like and humic-like compounds. FI: fluorescence index; BIX: biological index; HIX: humification index. AC/M: peak height ratio of Peaks A and C to Peak M; P/H: peak height ratio of Peak P to Peak H. -
Albéric P, Sarazin G, Michard G. 1996. Combined amino acid speciation in lake sediment and porewater (Aydat Lake, France). Aquatic Geochemistry, 2(1): 29–49. doi: 10.1007/BF00240852 Baker A, Bolton L, Newson M, et al. 2008. Spectrophotometric properties of surface water dissolved organic matter in an afforested upland peat catchment. Hydrological Processes, 22(13): 2325–2336. doi: 10.1002/hyp.6827 Berner R A. 1982. Burial of organic carbon and pyrite sulfur in the modern ocean: Its geochemical and environmental significance. American Journal of Science, 282(4): 451–473. doi: 10.2475/ajs.282.4.451 Berner R A. 1990. Global biogeochemical cycles of carbon and sulfur and atmospheric O2 over phanerozoic time. Chemical Geology, 84(1–4): 159 Burdige D J. 2001. Dissolved organic matter in Chesapeake Bay sediment pore waters. Organic Geochemistry, 32(4): 487–505. doi: 10.1016/S0146-6380(00)00191-1 Burdige D J, Kline S W, Chen Wenhao. 2004. Fluorescent dissolved organic matter in marine sediment pore waters. Marine Chemistry, 89(1–4): 289–311, Canfield D E, Jørgensen B B, Fossing H, et al. 1993. Pathways of organic carbon oxidation in three continental margin sediments. Marine Geology, 113(1–2): 27–40, Chen Meilian, Hur J. 2015. Pre-treatments, characteristics, and biogeochemical dynamics of dissolved organic matter in sediments: A review. Water Research, 79: 10–25. doi: 10.1016/j.watres.2015.04.018 Chin Y P, Traina S J, Swank C R, et al. 1998. Abundance and properties of dissolved organic matter in pore waters of a freshwater wetland. Limnology and Oceanography, 43(6): 1287–1296. doi: 10.4319/lo.1998.43.6.1287 Coble P. 2008. Cycling coloured carbon. Nature Geoscience, 1(9): 575–576. doi: 10.1038/ngeo294 Coble P G. 1996. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Marine Chemistry, 51(4): 325–346. doi: 10.1016/0304-4203(95)00062-3 Coble P G. 2007. Marine optical biogeochemistry: The chemistry of ocean color. Chemical Reviews, 107(2): 402–418. doi: 10.1021/cr050350+ Cuss C W, Guéguen C. 2016. Analysis of dissolved organic matter fluorescence using self-organizing maps: mini-review and tutorial. Analytical Methods, 8(4): 716–725. doi: 10.1039/C5AY02549D De Filippis P, Scarsella M. 2003. Oxidative desulfurization: Oxidation reactivity of sulfur compounds in different organic matrixes. Energy and Fuels, 17(6): 1452–1455. doi: 10.1021/ef0202539 Dittmar T, Koch B, Hertkorn N, et al. 2008. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnology and Oceanography: Methods, 6(6): 230–235. doi: 10.4319/lom.2008.6.230 Fellman J B, Hood E, Spencer R G M. 2010. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnology and Oceanography, 55(6): 2452–2462. doi: 10.4319/lo.2010.55.6.2452 Gan Shuchai, Schmidt F, Heuer V B, et al. 2020. Impacts of redox conditions on dissolved organic matter (DOM) quality in marine sediments off the River Rhône, Western Mediterranean Sea. Geochimica et Cosmochimica Acta, 276: 151–169. doi: 10.1016/j.gca.2020.02.001 Gan Shuchai, Wu Ying, Zhang Jing. 2016. Bioavailability of dissolved organic carbon linked with the regional carbon cycle in the East China Sea. Deep-Sea Research Part II: Topical Studies in Oceanography, 124: 19–28. doi: 10.1016/j.dsr2.2015.06.024 Guillemette F, von Wachenfeldt E, Kothawala D N, et al. 2017. Preferential sequestration of terrestrial organic matter in boreal lake sediments. Journal of Geophysical Research: Biogeosciences, 122(4): 863–874. doi: 10.1002/2016JG003735 Guo Weidong, Xu Jing, Wang Jiangping, et al. 2010. Characterization of dissolved organic matter in urban sewage using excitation emission matrix fluorescence spectroscopy and parallel factor analysis. Journal of Environmental Sciences, 22(11): 1728–1734. doi: 10.1016/S1001-0742(09)60312-0 Hansen A M, Kraus T E C, Pellerin B A, et al. 2016. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnology and Oceanography, 61(3): 1015–1032. doi: 10.1002/lno.10270 Hedges J I, Keil R G. 1995. Sedimentary organic matter preservation: an assessment and speculative synthesis. Marine Chemistry, 49(2–3): 81–115, Heuer V B, Aiello I W, Elvert M, et al. 2014. Report and preliminary results of R/V POSEIDON cruise POS450, DARCSEAS II–Deep subseafloor Archaea in the Western Mediterranean Sea: Carbon Cycle, Life Strategies, and Role in Sedimentary Ecosystems, Barcelona (Spain)–Malaga (Spain), April 2–13, 2013. Berichte, MARUM–Zentrum für Marine Umweltwissenschaften, Fachbereich Geowissenschaften, Universität Bremen. 305: 42 Heuer V B, Pohlman J W, Torres M E, et al. 2009. The stable carbon isotope biogeochemistry of acetate and other dissolved carbon species in deep subseafloor sediments at the northern Cascadia Margin. Geochimica et Cosmochimica Acta, 73(11): 3323–3336. doi: 10.1016/j.gca.2009.03.001 Holmer M, Kristensen E. 1996. Seasonality of sulfate reduction and pore water solutes in a marine fish farm sediment: the importance of temperature and sedimentary organic matter. Biogeochemistry, 32(1): 15–39. doi: 10.1007/BF00001530 Huang Shuangbing, Wang Yanxin, Ma Teng, et al. 2015. Linking groundwater dissolved organic matter to sedimentary organic matter from a fluvio-lacustrine aquifer at Jianghan Plain, China by EEM-PARAFAC and hydrochemical analyses. Science of the Total Environment, 529: 131–139. doi: 10.1016/j.scitotenv.2015.05.051 Huguet A, Vacher L, Relexans S, et al. 2009. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Organic Geochemistry, 40(6): 706–719. doi: 10.1016/j.orggeochem.2009.03.002 Ishii S K L, Boyer T H. 2012. Behavior of reoccurring PARAFAC components in fluorescent dissolved organic matter in natural and engineered systems: A critical review. Environmental Science and Technology, 46(4): 2006–2017. doi: 10.1021/es2043504 Jiang Fenghua, Yang Baijuan, Lee Frank Sen-Chun, et al. 2009. Multivariate analysis of fluorescence and source identification of dissolved organic matter in Jiaozhou Bay, China. Acta Oceanologica Sinica, 28(2): 60–72 Koch B P, Dittmar T. 2006. From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter. Rapid Communications in Mass Spectrometry, 20(5): 926–932. doi: 10.1002/rcm.2386 Koch B P, Dittmar T. 2016. From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter. Rapid Communications in Mass Spectrometry, 30(1): 250. doi: 10.1002/rcm.7433 Komada T, Schofield O M E, Reimers C E. 2002. Fluorescence characteristics of organic matter released from coastal sediments during resuspension. Marine Chemistry, 79(2): 81–97. doi: 10.1016/S0304-4203(02)00056-7 Krom M D, Sholkovitz E R. 1977. Nature and reactions of dissolved organic matter in the interstitial waters of marine sediments. Geochimica et Cosmochimica Acta, 41(11): 1565–1574. doi: 10.1016/0016-7037(77)90168-5 Lin H T, Cowen J P, Olson E J, et al. 2012. Inorganic chemistry, gas compositions and dissolved organic carbon in fluids from sedimented young basaltic crust on the Juan de Fuca Ridge flanks. Geochimica et Cosmochimica Acta, 85: 213–227. doi: 10.1016/j.gca.2012.02.017 Lin H T, Hsieh C C, Cowen J P, et al. 2015. Data report: dissolved and particulate organic carbon in the deep sediments of IODP Site U1363 near Grizzly Bare seamount. In: Proceedings of the Integrated Ocean Drilling Program, Volume 327. Tokyo: Integrated Ocean Drilling Program Management International, Inc. , Lin Y S, Koch B P, Feseker T, et al. 2017. Near-surface heating of young rift sediment causes mass production and discharge of reactive dissolved organic matter. Scientific Reports, 7(1): 44864. doi: 10.1038/srep44864 Lomstein B A, Jensen A G U, Hansen J W, et al. 1998. Budgets of sediment nitrogen and carbon cycling in the shallow water of Knebel Vig, Denmark. Aquatic Microbial Ecology, 14(1): 69–80. doi: 10.3354/ame014069 Lønborg C, Álvarez-Salgado X A, Davidson K, et al. 2010. Assessing the microbial bioavailability and degradation rate constants of dissolved organic matter by fluorescence spectroscopy in the coastal upwelling system of the Ría de Vigo. Marine Chemistry, 119(1–4): 121–129, Manciulea A, Baker A, Lead J R. 2009. A fluorescence quenching study of the interaction of Suwannee River fulvic acid with iron oxide nanoparticles. Chemosphere, 76(8): 1023–1027. doi: 10.1016/j.chemosphere.2009.04.067 McKnight D M, Boyer E W, Westerhoff P K, et al. 2001. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnology and Oceanography, 46(1): 38–48. doi: 10.4319/lo.2001.46.1.0038 Middelburg J J, Vlug T, Jaco F, et al. 1993. Organic matter mineralization in marine systems. Global and Planetary Change, 8(1–2): 47–58, Moini M, Jones B L, Rogers R M, et al. 1998. Sodium trifluoroacetate as a tune/calibration compound for positive- and negative-ion electrospray ionization mass spectrometry in the mass range of 100–4000 Da. Journal of the American Society for Mass Spectrometry, 9(9): 977–980. doi: 10.1016/S1044-0305(98)00079-8 Murphy K R, Butler K D, Spencer R G M, et al. 2010. Measurement of dissolved organic matter fluorescence in aquatic environments: An interlaboratory comparison. Environmental Science & Technology, 44(24): 9405–9412. doi: 10.1021/es102362t Murphy K R, Stedmon C A, Wenig P, et al. 2014. OpenFluor—an online spectral library of auto-fluorescence by organic compounds in the environment. Analytical Methods, 6(3): 658–661. doi: 10.1039/C3AY41935E Murphy K R, Stedmon C A, Graeber D, et al. 2013. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Analytical Methods, 5(23): 6557–6566. doi: 10.1039/c3ay41160e Ohno T. 2002. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environmental Science and Technology, 36(4): 742–746. doi: 10.1021/es0155276 Poncet-Legrand C, Cabane B, Bautista-Ortín A B, et al. 2010. Tannin oxidation: Intra-versus intermolecular reactions. Biomacromolecules, 11(9): 2376–2386. doi: 10.1021/bm100515e Poulin B A, Ryan J N, Aiken G R. 2014. Effects of iron on optical properties of dissolved organic matter. Environmental Science and Technology, 48(17): 10098–10106. doi: 10.1021/es502670r Rennert T, Gockel K F, Mansfeldt T. 2007. Extraction of water-soluble organic matter from mineral horizons of forest soils. Journal of Plant Nutrition and Soil Science, 170(4): 514–521. doi: 10.1002/jpln.200625099 Schmidt F, Elvert M, Koch B P, et al. 2009. Molecular characterization of dissolved organic matter in pore water of continental shelf sediments. Geochimica et Cosmochimica Acta, 73(11): 3337–3358. doi: 10.1016/j.gca.2009.03.008 Schmidt F, Koch B P, Elvert M, et al. 2011. Diagenetic transformation of dissolved organic nitrogen compounds under contrasting sedimentary redox conditions in the black sea. Environmental Science and Technology, 45(12): 5223–5229. doi: 10.1021/es2003414 Schmidt F, Koch B P, Goldhammer T, et al. 2017. Unraveling signatures of biogeochemical processes and the depositional setting in the molecular composition of pore water DOM across different marine environments. Geochimica et Cosmochimica Acta, 207: 57–80. doi: 10.1016/j.gca.2017.03.005 Schmidt F, Koch B P, Witt M, et al. 2014. Extending the analytical window for water-soluble organic matter in sediments by aqueous Soxhlet extraction. Geochimica et Cosmochimica Acta, 141: 83–96. doi: 10.1016/j.gca.2014.06.009 Schulz H D, Dahmke A, Schinzel U, et al. 1994. Early diagenetic processes, fluxes, and reaction rates in sediments of the South Atlantic. Geochimica et Cosmochimica Acta, 58(9): 2041–2060. doi: 10.1016/0016-7037(94)90284-4 Senesi N, Miano T M, Provenzano M R, et al. 1991. Characterization, differentiation, and classification of humic substances by fluorescence spectroscopy. Soil Science, 152(4): 259–271. doi: 10.1097/00010694-199110000-00004 Simoneit B R T, Sparrow M A. 2002. Dissolved organic carbon in interstitial waters from sediments of Middle Valley and Escanaba Trough, Northeast Pacific, ODP Legs 139 and 169. Applied Geochemistry, 17(11): 1495–1502. doi: 10.1016/S0883-2927(02)00114-2 Stedmon C A, Bro R. 2008. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnology and Oceanography: Methods, 6(11): 572–579. doi: 10.4319/lom.2008.6.572 Stedmon C A, Markager S. 2005. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnology and Oceanography, 50(2): 686–697. doi: 10.4319/lo.2005.50.2.0686 Tfaily M M, Hamdan R, Corbett J E, et al. 2013. Investigating dissolved organic matter decomposition in northern peatlands using complimentary analytical techniques. Geochimica et Cosmochimica Acta, 112: 116–129. doi: 10.1016/j.gca.2013.03.002 Valle J, Gonsior M, Harir M, et al. 2018. Extensive processing of sediment pore water dissolved organic matter during anoxic incubation as observed by high-field mass spectrometry (FTICR-MS). Water Research, 129: 252–263. doi: 10.1016/j.watres.2017.11.015 Wang Xiaona, Wu Ying, Bao Hongyan, et al. 2019. Sources, transport, and transformation of dissolved organic matter in a large river system: Illustrated by the Changjiang River, China. Journal of Geophysical Research:Biogeosciences, 124(12): 3881–3901. doi: 10.1029/2018JG004986 Weston N B, Porubsky W P, Samarkin V A, et al. 2006. Porewater stoichiometry of terminal metabolic products, sulfate, and dissolved organic carbon and nitrogen in estuarine intertidal creek-bank sediments. Biogeochemistry, 77(3): 375–408. doi: 10.1007/s10533-005-1640-1 Wolfe A P, Kaushal S S, Fulton J R, et al. 2002. Spectrofluorescence of sediment humic substances and historical changes of lacustrine organic matter provenance in response to atmospheric nutrient enrichment. Environmental Science and Technology, 36(15): 3217–3223. doi: 10.1021/es011215r Wu Weichao, Meador T, Hinrichs K U. 2018. Production and turnover of microbial organic matter in surface intertidal sediments. Organic Geochemistry, 121: 104–113. doi: 10.1016/J.ORGGEOCHEM.2018.04.006 Yamashita Y, Jaffé R, Maie N, et al. 2008. Assessing the dynamics of dissolved organic matter (DOM) in coastal environments by excitation emission matrix fluorescence and parallel factor analysis (EEM-PARAFAC). Limnology and Oceanography, 53(5): 1900–1908. doi: 10.4319/lo.2008.53.5.1900 Yamashita Y, Tanoue E. 2003. Chemical characterization of protein-like fluorophores in DOM in relation to aromatic amino acids. Marine Chemistry, 82(3–4): 255–271,doi: 10.1016/S0304-4203(03)00073-2 Yi Yueyuan, Zheng Airong, Guo Weidong, et al. 2014. Optical properties of estuarine dissolved organic matter isolated using cross-flow ultrafiltration. Acta Oceanologica Sinica, 33(4): 22–29. doi: 10.1007/s13131-014-0451-4 Zhuang Guang-Chao, Lin Yu-Shih, Elvert M, et al. 2014. Gas chromatographic analysis of methanol and ethanol in marine sediment pore waters: Validation and implementation of three pretreatment techniques. Marine Chemistry, 160: 82–90. doi: 10.1016/j.marchem.2014.01.011 Zsolnay A, Baigar E, Jimenez M, et al. 1999. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere, 38(1): 45–50. doi: 10.1016/S0045-6535(98)00166-0 -
 Gan Shuchai Supplementary materials2021 0318.docx
Gan Shuchai Supplementary materials2021 0318.docx

-



 下载:
下载: